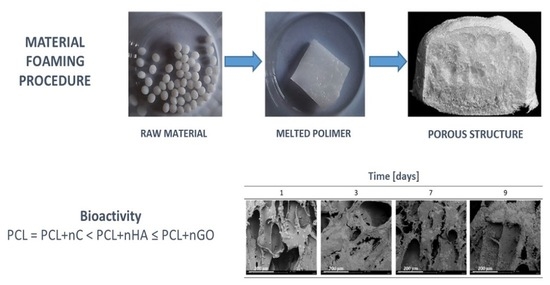
Foaming of PCL-Based Composites Using scCO2—Biocompatibility and Evaluation for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Nanohydroxyapatite
3.2. Nanocellulose
3.3. Nanographene Oxide
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salerno, A.; Domingo, C. Polycaprolactone foams prepared by supercritical CO2 batch foaming of polymer/organic solvent solutions. J. Supercrit. Fluids 2018, 143, 146–156. [Google Scholar] [CrossRef]
- Darłak, P.; Dudek, P. Materiały wysokoporowate—Metody wytwarzania i zastosowanie. Odlew—Nauka I Prakt. 2004, 4, 3. [Google Scholar]
- Tayton, E.; Purcell, M.; Aarvold, A.; Smith, J.; Kalra, S.; Briscoe, A.; Shakesheff, K.; Howdle, S.; Dunlop, D.; Oreffo, R. Supercritical CO2 fluid-foaming of polymers to increase porosity: A method to improve the mechanical and biocompatibility characteristics for use as a potential alternative to allografts in impaction bone grafting? Acta Biomater. 2012, 8, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Kosowska, K.; Krzysztoforski, J.; Henczka, M. Foaming of PCL-Based Composites Using scCO2: Structure and Physical Properties. Materials 2022, 15, 1169. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-X.; Peng, H.-H.; Guan, Y.-X.; Yao, S.-J. Morphological study on the pore growth profile of poly(ε-caprolactone) bi-modal porous foams using a modified supercritical CO2 foaming process. J. Supercrit. Fluids 2018, 143, 72–81. [Google Scholar] [CrossRef]
- Di Di Maio, E.; Iannace, S.; Di, Y.; Del Giacomo, E.; Nicolais, L. Heterogeneous bubble nucleation in PCL/clay nanocomposite foams. Plast. Rubber Compos. 2003, 32, 313–317. [Google Scholar] [CrossRef]
- Marrazzo, C.; Di Maio, E.; Iannace, S. Conventional and Nanometric Nucleating Agents in Poly(ε-caprolactone) Foaming: Crystals vs. Bubbles Nucleation. Polym. Eng. Sci. 2008, 48, 336–344. [Google Scholar] [CrossRef]
- Mi, H.Y.; Jing, X.; Peng, J.; Salick, M.R.; Peng, X.F.; Turng, L.S. Poly(ε-caprolactone) (PCL)/cellulose nano-crystal (CNC) nanocomposites and foams. Cellulose 2014, 21, 2727–2741. [Google Scholar] [CrossRef]
- Reignier, J.; Huneault, M.A. Preparation of interconnected poly(ε-caprolactone) porous scaffolds by a combination of polymer and salt particulate leaching. Polymer 2006, 47, 4703–4717. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.Q.; Huang, H.X. Formation mechanism and tuning for bi-modal cell structure in polystyrene foams by synergistic effect of temperature rising and depressurization with supercritical CO2. J. Supercrit. Fluids 2016, 109, 177–185. [Google Scholar] [CrossRef]
- Rouholamin, D.; Van Grunsven, W.; Reilly, G.C.; Smith, P.J. Morphological effects of porous poly-d,l-lactic acid/hydroxyapatite scaffolds produced by supercritical CO2 foaming on their mechanical performance. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2016, 230, 761–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, X.; Guan, Y.; Yao, S. Bi-/multi-modal pore formation of PLGA/hydroxyapatite composite scaffolds by heterogeneous nucleation in supercritical CO2 foaming. Chin. J. Chem. Eng. 2018, 26, 207–212. [Google Scholar] [CrossRef]
- Tsimpliaraki, A.; Tsivintzelis, I.; Marras, S.I.; Zuburtikudis, I.; Panayiotou, C. Foaming of PCL/clay nanocomposites with supercritical CO2 mixtures: The effect of nanocomposite fabrication route on the clay dispersion and the final porous structure. J. Supercrit. Fluids 2013, 81, 86–91. [Google Scholar] [CrossRef]
- Xue, W.; Chen, P.; Wang, F.; Wang, L. Melt spinning of nano-hydroxyapatite and polycaprolactone composite fibers for bone scaffold application. J. Mater. Sci. 2019, 54, 8602–8612. [Google Scholar] [CrossRef]
- Moghadam, M.Z.; Hassanajili, S.; Esmaeilzadeh, F.; Ayatollahi, M.; Ahmadi, M. Formation of porous HPCL/LPCL/HA scaffolds with supercritical CO2 gas foaming method. J. Mech. Behav. Biomed. Mater. 2017, 69, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Borkotoky, S.S.; Dhar, P.; Katiyar, V. Biodegradable poly (lactic ac-id)/Cellulose nanocrystals (CNCs) composite microcellular foam: Effect of nanofillers on foam cellular morphology, thermal and wettability behavior. Int. J. Biol. Macromol. 2018, 106, 433–446. [Google Scholar] [CrossRef]
- Gedler, G.; Antunes, M.; Velasco, J.I. Effects of graphene nanoplatelets on the morphology of polycarbonate–graphene composite foams prepared by supercritical carbon dioxide two-step foaming. J. Supercrit. Fluids 2015, 100, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Chen, W.; Jia, Y.; Bai, S.; Wang, Q. Fabrication of poly (vinyl alcohol)/graphene nanocomposite foam based on solid state shearing milling and supercritical fluid technology. Mater. Des. 2017, 134, 121–131. [Google Scholar] [CrossRef]
- Kosowska, K.; Henczka, M. The influence of supercritical foaming conditions on properties of polymer scaffolds for tissue engineering. Chem. Process Eng. 2017, 38, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Sawicka, K.; Kosowska, K.; Henczka, M. Application of porogenes in pro-duction of porous polymers by supercritical foaming. Chem. Process Eng. 2019, 40, 115–122. [Google Scholar]
- Guarino, V.; Ambrosio, L. Properties of biomedical foams for tissue engineering applications. In W Biomedical Foams for Tissue Engineering Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 40–70. [Google Scholar] [CrossRef]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Chen, C.X.; Liu, Q.Q.; Xin, X.; Guan, Y.X.; Yao, S.J. Pore formation of poly(ε-caprolactone) scaffolds with melting point reduction in supercritical CO2 foaming. J. Supercrit. Fluids 2016, 117, 279–288. [Google Scholar] [CrossRef]
- Kramschuster, A.; Turng, L.S. Fabrication of Tissue Engineering Scaffolds. In W Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Frohlich, M.; Grayson, W.; Wan, L.; Marolt, D.; Drobnic, M.; Vunjak- Novakovic, G. Tissue Engineered Bone Grafts: Biological Requirements, Tissue Culture and Clinical Relevance. Curr. Stem Cell Res. Ther. 2008, 3, 254–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salerno, A.; Zeppetelli, S.; Di Maio, E.; Iannace, S.; Netti, P.A. Novel 3D porous multi-phase composite scaffolds based on PCL, thermoplastic zein and ha prepared via supercritical CO2 foaming for bone regeneration. Compos. Sci. Technol. 2010, 70, 1838–1846. [Google Scholar] [CrossRef] [Green Version]
- Salerno, A.; Netti, P.A. Introduction to biomedical foams. Biomed. Foam. Tissue Eng. Appl. 2014, 3–39. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and os-teogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Velasco, M.A.; Narvaez-Tovar, C.A.; Garzon-Alvarado, D.A. Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. Bio-Med Res. Int. 2015, 2015, 729076. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Mallick, S.; Tripathi, S.; Srivastava, P. Advancement in Scaffolds for Bone Tissue Engineering: A Review. IOSR J. Pharm. Biol. Sci. 2015, 10, 2319–7676. [Google Scholar] [CrossRef]
- White, L.J.; Hutter, V.; Tai, H.; Howdle, S.M.; Shakesheff, K.M. The effect of processing variables on morphological and mechanical properties of supercritical CO2 foamed scaffolds for tissue engineering. Acta Biomater. 2012, 8, 61–71. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Khan, Y.; Yaszemski, M.J.; Mikos, A.G.; Laurencin, C.T. Tissue engineering of bone: Material and matrix considerations. J. Bone Jt. Surg. Ser. A 2008, 90 (Suppl. S1), 36–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Park, I.-K.; Hoshiba, T.; Jiang, H.-L.; Choi, Y.-J.; Akaike, T.; Cho, C.-S. Design of artificial extracellular matrices for tissue engineering. Prog. Polym. Sci. 2011, 36, 238–268. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Pol-ycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]
- Shim, M.; Kam, N.W.S.; Chen, R.J.; Li, Y.; Dai, H. Functionalization of Carbon Nanotubes for Biocompatibility and Biomolecular Recognition. Nano Lett. 2002, 2, 285–288. [Google Scholar] [CrossRef]
- Salerno, A.; Di Maio, E.; Iannace, S.; Netti, P.A. Solid-state supercritical CO2 foaming of PCL and PCL-HA nano-composite: Effect of composition, thermal history and foaming process on foam pore structure. J. Supercrit. Fluids 2011, 58, 158–167. [Google Scholar] [CrossRef]
- Jenkins, M.J.; Harrison, K.L.; Silva, M.M.C.G.; Whitaker, M.J.; Shakesheff, K.M.; Howdle, S.M. Characterisation of microcellular foams produced from semi-crystalline PCL using supercritical carbon dioxide. Eur. Polym. J. 2006, 42, 3145–3151. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]







| Property | Parameter | The Range of Variability | Application | References |
|---|---|---|---|---|
| Morphology | Porosity | 80% | Biomedical engineering | [28,29,30,31,32,42] |
| Pore size | 5 μm | Newly formed blood vessels | [36] | |
| 5–15 μm | Connective tissue cells in the growth phase | |||
| 20 μm | Hepatocytes | |||
| 20–125 μm | The skin of an adult human | |||
| 100–350 μm | Bone tissue | |||
| >500 μm | Fibrous vascular tissue | |||
| Micropores | Tissue engineering | [37] | ||
| 100 μm | Bone tissue | [28] | ||
| 450 μm | Bone tissue | [43] | ||
| ≤100 μm | Connective tissue | |||
| Micropores (<50 μm) and macropores (150–300 μm) | Fibrous cartilage tissue | |||
| <100 μm | Bone tissue | [26] | ||
| 100–500 μm | Blood vessels | |||
| 200–350 μm | Bone tissue | [30] | ||
| 100–350 μm | Bone tissue | [36] | ||
| Mechanical | Young’s modulus | 7–30 GPa | A creature packed with bones | [25,29,39] |
| 0.02–0.8 GPa | Bone spongy creature | |||
| Mechanical strength | 100–230 MPa | A creature packed with bones | ||
| 2–12 MPa | Bone spongy creature | |||
| Biological | Biocompatibility | Optimal biotolerance, non-toxicity, no genetic mutations in the surrounding cells and no inflammation | Biomedical engineering | [24,29,33,40,41,44] |
| Biodegradability | The rate of degradation of the implanted material was adjusted to the rate of regeneration of damaged tissue | Biomedical engineering | [24,29,33] |
| MTT | Presto Blue | ||
|---|---|---|---|
| CELL VIABILITY [%] | |||
| CONTROL | 100 (±0.10) | 100 (±0.02) | |
| P [MPa] | 9 | 94.6 (±0.11) | 95.2 (±0.15) |
| 18 | 97.5 (±0.05) | 97.7 (±0.05) | |
| t [h] | 0.5 | 93.2 (±0.01) | 91.3 (±0.02) |
| 6 | 98.0 (±0.03) | 87.3 (±0.25) | |
| T [°C] | 50 | 84.0 (±0.06) | 91.0 (±0.10) |
| 100 | 87.0 (±0.04) | 95.0 (±0.01) | |
| D | Dslow | 91.4 (±0.01) | 84.2 (±0.12) |
| Dfast | 87.8 (±0.02) | 87.6 (±0.02) | |
| C [%(w/w)] | MTT | Presto Blue | ||
|---|---|---|---|---|
| CELL VIABILITY [%] | ||||
| CONTROL | 100 (±0.10) | 100 (±0.02) | ||
| P [MPa] | 9 | 1 | 80 (±0.11) | 78.0 (±0.25) |
| 5 | 78 (±0.05) | 85.3 (±0.04) | ||
| 12 | 82 (±0.01) | 80.0 (±0.03) | ||
| 18 | 1 | 89 (±0.03) | 90.5 (±0.01) | |
| 5 | 86.5 (±0.06) | 82.3 (±0.03) | ||
| 12 | 84.5 (±0.04) | 85.6 (±0.11) | ||
| t [h] | 1 | 1 | 87.5 (±0.01) | 80.8 (±0.01) |
| 5 | 92.4 (±0.02) | 98.4 (±0.02) | ||
| 12 | 95.0 (±0.10) | 88.2 (±0.11) | ||
| 4 | 1 | 98.0 (±0.11) | 85.3 (±0.02) | |
| 5 | 93.0 (±0.05) | 87.6 (±0.06) | ||
| 12 | 88.0 (±0.01) | 82.6 (±0.02) | ||
| T [°C] | 50 | 1 | 75.0 (±0.03) | 80.5 (±0.10) |
| 5 | 85.0 (±0.06) | 82.6 (±0.05) | ||
| 12 | 82.5 (±0.04) | 80.6 (±0.02) | ||
| 100 | 1 | 93.5 (±0.01) | 92.5 (±0.11) | |
| 5 | 87.0 (±0.02) | 90.5 (±0.05) | ||
| 12 | 85.5 (±0.10) | 86.2 (±0.02) | ||
| C [%(w/w)] | MTT | Presto Blue | ||
|---|---|---|---|---|
| CELL VIABILITY [%] | ||||
| CONTROL | 100 (±0.10) | 100 (±0.02) | ||
| P [MPa] | 9 | 1 | 98.7 (±0.11) | 88.2 (±0.04) |
| 5 | 92.3 (±0.05) | 80.4 (±0.05) | ||
| 12 | 95.0 (±0.01) | 80.6 (±0.10) | ||
| 18 | 1 | 88.4 (±0.03) | 85.4 (±0.11) | |
| 5 | 87.5 (±0.06) | 85.4 (±0.05) | ||
| 12 | 85.5 (±0.04) | 90.4 (±0.10) | ||
| t [h] | 1 | 1 | 89.4 (±0.01) | 92.4 (±0.04) |
| 5 | 84.5 (±0.02) | 90.4 (±0.10) | ||
| 12 | 86.4 (±0.10) | 88.2 (±0.02) | ||
| 4 | 1 | 96.4 (±0.11) | 92.4 (±0.01) | |
| 5 | 96.7 (±0.05) | 90.4 (±0.06) | ||
| 12 | 88.5 (±0.01) | 80.4 (±0.05) | ||
| T [°C] | 50 | 1 | 78.4 (±0.03) | 82.6 (±0.11) |
| 5 | 77.6 (±0.06) | 80.4 (±0.03) | ||
| 12 | 82.5 (±0.04) | 81.3 (±0.10) | ||
| 100 | 1 | 86.4 (±0.01) | 82.4 (±0.03) | |
| 5 | 88.7 (±0.02) | 81.5 (±0.06) | ||
| 12 | 74.55 (±0.10) | 82.5 (±0.01) | ||
| C [%(w/w)] | MTT | Presto Blue | ||
|---|---|---|---|---|
| CELL VIABILITY [%] | ||||
| CONTROL | 100 (±0.02) | 100 (±0.10) | ||
| P [MPa] | 9 | 0.2 | 95.0 (±0.11) | 88.2 (±0.02) |
| 0.5 | 92.0 (±0.05) | 90.4 (±0.01) | ||
| 1 | 88.0 (±0.01) | 90.4 (±0.05) | ||
| 1.5 | 87.0 (±0.03) | 85.4 (±0.03) | ||
| 18 | 0.2 | 96.4 (±0.06) | 86.4 (±0.10) | |
| 0.5 | 86.7 (±0.04) | 84.5 (±0.06 | ||
| 1 | 89.2 (±0.01) | 90.4 (±0.10) | ||
| 1.5 | 76.4 (±0.02) | 92.1 (±0.06) | ||
| t [h] | 1 | 0.2 | 88.7 (±0.10) | 90.5 (±0.02) |
| 0.5 | 89.6 (±0.11) | 87.5 (±0.01) | ||
| 1 | 82.6 (±0.05) | 86.5 (±0.03) | ||
| 1.5 | 74.2 (±0.01) | 80.4 (±0.02) | ||
| 4 | 0.2 | 82.6 (±0.03) | 86.2 (±0.01) | |
| 0.5 | 86.2 (±0.06) | 80.2 (±0.05) | ||
| 1 | 78.5 (±0.04) | 78.4 (±0.03) | ||
| 1.5 | 98.5 (±0.01) | 82.5 (±0.02) | ||
| T [°C] | 50 | 0.2 | 92.6 (±0.02) | 80.2 (±0.01) |
| 0.5 | 88.4 (±0.10) | 89.1 (±0.06) | ||
| 1 | 82.6 (±0.01) | 90.2 (±0.01) | ||
| 1.5 | 86.4 (±0.03) | 80.5 (±0.02) | ||
| 100 | 0.2 | 88.6 (±0.06) | 86.4 (±0.05) | |
| 0.5 | 89.6 (±0.04) | 82.6 (±0.01) | ||
| 1 | 87.5 (±0.01) | 84.5 (±0.06) | ||
| 1.5 | 84.6 (±0.02) | 87.3 (±0.01) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosowska, K.; Krzysztoforski, J.; Henczka, M. Foaming of PCL-Based Composites Using scCO2—Biocompatibility and Evaluation for Biomedical Applications. Materials 2022, 15, 3858. https://doi.org/10.3390/ma15113858
Kosowska K, Krzysztoforski J, Henczka M. Foaming of PCL-Based Composites Using scCO2—Biocompatibility and Evaluation for Biomedical Applications. Materials. 2022; 15(11):3858. https://doi.org/10.3390/ma15113858
Chicago/Turabian StyleKosowska, Katarzyna, Jan Krzysztoforski, and Marek Henczka. 2022. "Foaming of PCL-Based Composites Using scCO2—Biocompatibility and Evaluation for Biomedical Applications" Materials 15, no. 11: 3858. https://doi.org/10.3390/ma15113858
APA StyleKosowska, K., Krzysztoforski, J., & Henczka, M. (2022). Foaming of PCL-Based Composites Using scCO2—Biocompatibility and Evaluation for Biomedical Applications. Materials, 15(11), 3858. https://doi.org/10.3390/ma15113858