3.1. Micro-Flotation Study
The effect of pH on the recovery rates is presented in
Figure 3a. The recovery of scheelite increased and then decreased as pH increases with the addition of sodium silicate as an inhibitor, while calcite showed a continuous decreasing trend and stabilized. The difference between scheelite and calcite recoveries was the largest at pH = 9 ± 0.2, and, thus, was subsequently selected as the optimal pH to study the effect of Na
2SiO
3 dosage on the recoveries of both minerals. The effect of Na
2SiO
3 dosage on the recovery rates is indicated in
Figure 3b. The scheelite recovery decreased gradually with the increase of Na
2SiO
3 dosage; it shows that when too much Na
2SiO
3 is used, it will also adsorb with scheelite, thus reducing the FR of scheelite [
25]. Relatively speaking, the inhibition effect of Na
2SiO
3 on calcite was more obvious. Among all the Na
2SiO
3 dosage contents, 1.2 g/L showed a better separation effect (As shown by the dotted line in the
Figure 3b). The FR of the two minerals differed by 40.03%; however, it is insufficient to effectively separate scheelite and calcite. Therefore, the separation effect of both minerals was enhanced by adding metal ions.
The effect of adding MnCl
2 at different stages on the flotation recoveries of the two minerals is indicated in
Figure 4 and
Figure 5, and
Figure 4b represents the recovery rates when MnCl
2 was added first, followed by Na
2SiO
3. The scheelite recovery first increased and then decreased significantly with the increase of pH, while the recovery of calcite gradually decreased and then flattened out. It can be seen that the best separation efficiency of the two minerals was achieved at pH = 9 ± 0.2. On the other hand, when the dosing sequence was Na
2SiO
3 followed by MnCl
2, the recovery of scheelite tended to increase gradually as pH increases, reaching the maximum at pH = 9 ± 0.2, after which it started decreasing. In contrast, the recovery of calcite gradually decreased and then leveled off, similar to that in the other dosing sequence with MnCl
2 + Na
2SiO
3.
Furthermore, the relationship between Mn
2+ concentration and mineral FR was also investigated. For the target mineral scheelite, it is obvious from
Figure 4a that the recovery of scheelite firstly increases with the addition sequence of MnCl
2 + Na
2SiO
3, and then decreases continuously with the increase of the Mn
2+ concentration. However, when Na
2SiO
3 was added first, the recovery of scheelite increased first and then leveled off at a concentration of Mn
2+ of 5 × 10
−4 mol/L (
Figure 5a). The separation efficiency of scheelite and calcite under this condition was the best, with a difference of 82.84% in recovery.
As for the calcite, after the addition of MnCl2, its FR was lower than the condition of Na2SiO3 alone, regardless of the addition order, indicating that the addition of MnCl2 contributes to the selective inhibition of calcite by Na2SiO3. As the Mn2+ dosage increases, the recovery of calcite gradually decreased and then leveled off. Therefore, the dosing sequence of Na2SiO3 + MnCl2 can separate the two minerals more effectively.
3.2. Zeta Potential Analysis
This assay can be used to analyze the adsorption behavior of reagents on the surface of mineral particles during flotation and the effect on the surface charge of minerals [
26]. The effect of pH on the surface zeta potential of mineral particles of scheelite and calcite is given in
Figure 6.
Figure 6a indicates that the zero electric point is not observed in scheelite throughout the pH interval and the surface potentials are negative [
12]. The iso-electric point (IEP) of the surface of calcite was obtained at around pH = 9.5 (
Figure 6b), which was consistent with previous literature studies [
13,
27].
Obviously, when NaOL interacts with minerals, the potentials all tend to show a negative shift (line 2 in
Figure 6), indicating that the interaction of the oleate ion (RCOO
−) with the mineral surface in the anion collector NaOL leads to a transfer in the negative direction, as evidenced by previous studies by some scholars [
26,
28]. In contrast, when Na
2SiO
3 was added (line 3 in
Figure 6), The mineral particle surface potentials of both scheelite and calcite are shifted in the positive direction; however, the shift of the scheelite potential is smaller. The results of the previous flotation tests also confirm this, indicating that sodium silicate would interact more with the calcite surface; thus, it prevents NaOL from reacting with its surface and inhibits its floating [
27].
When Mn
2+ was added first (line 4 in
Figure 6a), The scheelite potential was positively shifted by 5.5 mV at pH = 9 compared with the case of adding only Na
2SiO
3. The increase of sodium silicate adsorption may decrease the adsorption of NaOL on the mineral surface. On the other side, when the MnCl
2 is added later (line 5 in
Figure 6a), the scheelite potential is clearly the most negatively shifted compared to all other dosing conditions and shifted in the negative direction by 4.5 mV when only sodium silicate was added. Referring to the flotation test results, which showed an increase in recovery compared with the addition of Na
2SiO
3 only, it is obvious that the post-addition of Mn
2+ promotes the activity of the scheelite surface and causes more oleate ions to react with the surface of scheelite by adsorption, thus contributing to the negative shift trend of surface potential.
After adding Mn
2+ first (line 4 in
Figure 6b), the calcite surface potential is positively shifted by 8.5 mV at pH = 9 when Mn
2+ was added first, more than that when only sodium silicate was added, due to the lower adsorption of oleate ions. The calcite surface potential was slightly shifted in the negative direction when Mn
2+ was added later (line 5 in
Figure 6b), more than when Mn
2+ was added first. The recovery was slightly higher when Mn
2+ was added first. The addition of Mn
2+ first provides active sites for sodium silicate, resulting in a slight enhancement of the calcite surface hydrophilicity. Therefore, the dosing sequence of adding Na
2SiO
3 first and then Mn
2+ was chosen as the best condition.
3.3. UV–Vis Analysis
The effect of adding different agents and varying dosages is presented in
Figure 7a. The addition of sodium silicate resulted in an inhibition effect in both minerals; however, the inhibition effect on calcite was more obvious. The adsorption capacity of NaOL on both mineral surfaces showed a negative correlation with the dosage of Na
2SiO
3. The increase of sodium silicate dosage makes it impossible to achieve FS of two minerals.
The effect of increasing the Mn
2+ concentration on the adsorption amount of NaOL when MnCl
2 was added first is shown in
Figure 7b. An increasing trend was observed with the increasing dosage of Mn
2+ when MnCl
2 was added first, compared to that without MnCl
2 addition. This may be due to the fact that at low Mn
2+ concentrations, NaOL will preferentially adsorb on the scheelite surface before Na
2SiO
3 due to the poor adsorption of sodium silicate itself to scheelite, causing an increase in adsorption. On the other hand, the increase of Mn
2+ dosage led to more active sites of Mn
2+ adsorption on the scheelite surface, and, thus, the adsorption of Na
2SiO
3 also increased, which increased the hydrophilicity of the surface. In addition, the adsorption of NaOL on scheelite showed a decreasing trend, indicating that the increase of Mn
2+ dosage would inhibit the flotation of scheelite. In addition, the adsorption of NaOL on calcite also showed a decreasing trend, leading to the inability of the two minerals to be separated.
As shown in
Figure 7c, the addition of MnCl
2 after Na
2SiO
3 increased the adsorption of C
18H
33NaO
2 on the scheelite surface gradually as the Mn
2+ concentration increases. The NaOL content of the calcite surface gradually decreased and did not vary much under the two dosing orders, which was consistent with the results of the flotation test and zeta potential. At the dosage of Mn
2+ of 5 × 10
−4 mol/L, the difference in the C
18H
33NaO
2 adsorption amount between the two minerals reached 2.306 × 10
–6 mol/g. It indicates that the dosing sequence of adding Mn
2+ after sodium silicate can facilitate the FS of the two minerals.
3.4. FTIR Analysis
The IR spectral method is used to study the nature and phenomena of adsorption of substances at the solid–liquid interface to study the nature of adsorption of flotation chemicals on mineral surfaces during flotation, as well as the orientation of adsorbed substances and detailed information on adsorption/resolution equilibria and substance formation [
29]. The mechanism of the action of the agents on the surface of the two minerals under the conditions of two incorporation points of manganese ions was analyzed by infrared spectroscopy.
The IR spectra before and after the addition of agents are displayed in
Figure 8. In the presence of added sodium oleate, new absorption peaks were observed at 2920.60 cm
−1 and 2850.71 cm
−1, indicating the adsorption of C
18H
33NaO
2 onto the scheelite surface, respectively, and the absorption peaks at 1574.65 cm
−1, 1538.65 cm
−1, and 1468.19 cm
−1 due to the symmetric and asymmetric stretching vibrations of carboxyl [
30]. It indicates that NaOL is adsorbed on the scheelite surface. The absorption peaks at 2920.36 cm
−1 and 2850.55 cm
−1 were attributed to -CH
2-symmetric stretching peaks after the interaction of Na
2SiO
3 and NaOL with minerals [
31]. After adding Mn
2+ in a different order, a new peak appeared in the IR spectrum at 3421.77 cm
−1 on the scheelite surface, due to the interaction of MnCl
2 with the surface of the scheelite mineral. When Mn
2+ was added after sodium silicate, the absorption peaks at 2921.98 cm
−1 and 2850.56 cm
−1, because of the antisymmetric stretching peak of alkyl CH
2, and the absorption peaks at 1559.63 cm
−1 and 1446.24 cm
−1, are attributed to the -COO absorption vibration peak. Meanwhile, the absorption peaks of alkyl CH
2 and COO- appearing on the scheelite surface are significantly enhanced in intensity compared with those under other agent regimes. The results showed that Mn
2+ added after sodium silicate could adsorb onto the scheelite surface and enhance its Mn
2+ active site; thus, the adsorption capacity of NaOL on the scheelite surface was enhanced.
After adding NaOL (
Figure 8b), the absorption peaks at 2920.97 cm
−1 and 2851.04 cm
−1 because of the antisymmetric stretching peak of CH
2. The absorption peak of calcite at 1443.48 cm
−1 is shifted to 1424.30 cm
−1. After the addition of Mn
2+ after sodium silicate, the absorption peaks on the calcite surface at 2921.02 cm
−1 and 2851.25 cm
−1, because of the antisymmetric stretching peaks of alkyl CH
2, and the peaks at 1572.53 cm
−1 and 1534.69 cm
−1 were attributed to the COO-absorption vibration peaks. In comparison with the IR spectra of calcite after the action of the other three agent regimes, the absorption peaks of alkyl CH
2 and COO- were also present on the calcite surface, but their intensities were greatly weakened. Combined with the results of the adsorption amount test, and obviously, after the addition of Na
2SiO
3, they preferentially adsorb with calcite, and then after the addition of manganese chloride, there is more free Mn
2+ in the solution due to the physical and chemical adsorption of calcite surface with sodium silicate, followed by the inability to adsorb Mn
2+. After adding sodium oleate, more oleate is generated by the reaction between Mn
2+ and sodium oleate, which makes the calcite-weak sodium oleate absorption peaks appear on the surface.
The IR spectra of the two mineral surfaces were analyzed together, and the two dosing orders of MnCl2 had different effects on the adsorption of inhibitors and traps on the surfaces of the two minerals. The dosing sequence of adding MnCl2 after Na2SiO3 can enhance the adsorption capacity of NaOL to scheelite and reduce the adsorption capacity of NaOL to calcite, thus enabling the two minerals to be separated.
3.5. XPS Analysis
The XPS spectra of scheelite under different dosing sequences (
Figure 9a) show that the characteristic peaks of Mn and Si appear under two different dosing sequences (I, MnCl
2 + Na
2SiO
3 + NaOL; II, Na
2SiO
3 + MnCl
2 + NaOL), which led to the conclusion that Mn and Si would adsorb to the surface of scheelite under these two conditions. However, the peak intensity of C1s in II was obviously larger than that of I. The atomic concentration statistics (
Figure 9b) of C in the II order increased by 9.3% compared to those of I. Moreover, the atomic concentration of Si under the II condition is 1.8%, which is 1.1% lower than that of I, indicating that the I order can lead to more sodium silicate acting on the scheelite surface, leading to the enhancement of hydrophilicity on the scheelite surface. This may be because in condition I (when Mn
2+ was added first), Mn
2+ with more active sites adsorbs on the surface, and subsequently, after adding sodium silicate, reacts with silicate to generate more metal silicates adsorbed on the scheelite surface. In condition II (when sodium silicate was added first), sodium silicate is less selective, and thus less adsorbed on scheelite. After adding MnCl
2, Mn
2+ acts on the surface of minerals, and when sodium oleate is added, it can better act with scheelite to strengthen the flotation effect of scheelite.
The XPS spectra of calcite under different dosing sequences (
Figure 10a) show that the characteristic peaks of Mn and Si were generated under both conditions. Comparing the intensity of the peaks under the two dosing orders, the intensity of the peaks of C1s under the I order is lower than that in II. The atomic concentration statistics (
Figure 10b) reveal that C atomic concentration in the I order is 3.1% lower than that of II, while the concentration of Si atoms is 1% more than that of II. It can be seen that MnCl
2 acts with calcite first before Na
2SiO
3, which can reduce the floatability of minerals. However, when MnCl
2 was added first, resulting in the absence of selectivity of sodium silicate for scheelite and calcite, the recovery of calcite was lower under the I sequence. It is not possible to separate scheelite and calcite by this dosing sequence. Therefore, the separation of the two minerals under the dosing sequence of Minerals + Na
2SiO
3 + MnCl
2 + NaOL was better than that of sodium silicate alone.
The high-resolution XPS spectra of the Ca2p of scheelite and calcite interacting with pharmaceuticals under different dosing sequences were analyzed by peak fitting and separation. It can be observed from
Figure 11a that the peaks appearing at the binding energies of 346.94 eV and 350.49 eV can be assigned to Ca2p3/2 and Ca2p1/2, respectively [
31]. The binding energies (BE) of Ca2p3/2 and Ca2p1/2 on the surface of scheelite after the action of MnCl
2 + Na
2SiO
3 + NaOL were both shifted by 0.15 eV and after, the actions of Na
2SiO
3 + MnCl
2 + NaOL were both shifted by 0.13 eV. The Ca2p BE on the surface of the scheelite had some offset, but the offset value was smaller than the error value, which indicated that the effect of chemicals on the Ca2p orbitals on the surface of the scheelite was small under the two dosing sequences.
The calcite also presented a similar spectrum for Ca2p. As can be observed from
Figure 11b, the peaks Ca2p3/2 and Ca2p1/2 appeared at the binding energies of 346.90 eV and 350.45 eV, respectively [
5]. The BE shift of Ca2p3/2 on the calcite surface after the action of MnCl
2 + Na
2SiO
3 + NaOL was 0.52 eV, and that of Ca2p1/2 was 0.42 eV. Similarly, the BE shift of Ca2p3/2 on the calcite surface after the action of Na
2SiO
3 + MnCl
2 + NaOL was 0.44 eV, and there was one for Ca2p1/2. The shift after the action of MnCl
2 + Na
2SiO
3 + NaOL was more significant, indicating that the chemisorption was stronger under this dosing sequence, the results of previous flotation tests also confirm this. The Ca2p BE on the calcite surface also shifted after the action with the agent, and the shifted values were larger than the instrumental error values, indicating that the agent can react with the calcite surface.
3.6. Solution Chemistry Calculation Analysis
The effect of pH on the species formation and content of Mn
2+, silicates in the Mn
2+, and the silicate solution system is indicated in
Figure 12. Based on
Figure 12, the main chemical equations for the chemical equilibrium of Mn
2+ and the silicate in a solution can be deduced as follows:
Figure 12a shows the gradual hydrolysis of Mn
2+ in the solution and the generation of four manganese-containing components: MnOH
+,
,
, and
. When the pH < 9.29, Mn
2+ dominates the composition. After increasing the pH,
,
, and
dominate the system.
Figure 12b shows that there are three main components in the Na
2SiO
3 solution. Si(OH)
4 dominates when the pH < 9.6, and
and
dominate when the pH < 12.8. When MnCl
2 is added after Na
2SiO
3, the hydrolysis of Mn
2+ consumes lots of OH
−. Consequently, the concentration of H
+ in the solution increases and Equations (8) and (9) move in the opposite direction, leading to the production of more Si(OH)
4. Si(OH)
4 is the active component that inhibits calcite flotation since it selectively acts on calcite and interferes with the adsorption of NaOL on its surface, thus reducing the recovery during calcite flotation [
23,
27]. When MnCl
2 is added before Na
2SiO
3, Mn
2+ is pre-sorbed on the surface of both minerals, and when Na
2SiO
3 is subsequently added, the silicate hydrolysis products interact with the mineral surface through physical adsorption, resulting in both scheelite and calcite being inhibited from floating.
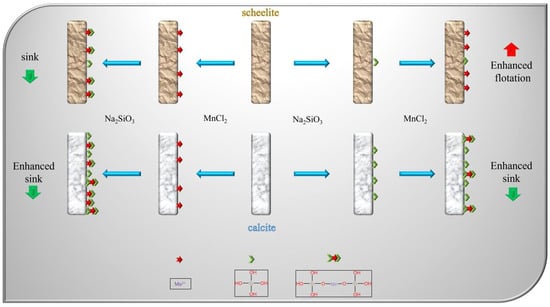
The mechanism of the interaction between two different addition points of MnCl
2 and mineral surfaces is shown in
Figure 13. Under the additional order of MnCl
2 + Na
2SiO
3, Mn
2+ will be adsorbed on both mineral surfaces due to its lack of selectivity, increasing the active sites on both minerals surfaces. This resulted in enhanced adsorption of sodium silicate on both minerals surfaces., which led to the enhancement of hydrophilicity on both minerals surfaces. This inhibited the minerals, and separation could not be achieved. In contrast, under the dosing sequence of Na
2SiO
3+ MnCl
2, sodium silicate was added first, and Na
2SiO
3 selectively adsorbed on calcite surface, while it adsorbed less on scheelite. With the subsequential addition of MnCl
2, Mn
2+ reacted with the surface of scheelite and adsorbed on its surface, thus increasing the active site of NaOL on its surface. It also reacted with free sodium silicate in the pulp to form a more selective metal silicate; as a result, the inhibition effect on calcite was enhanced.