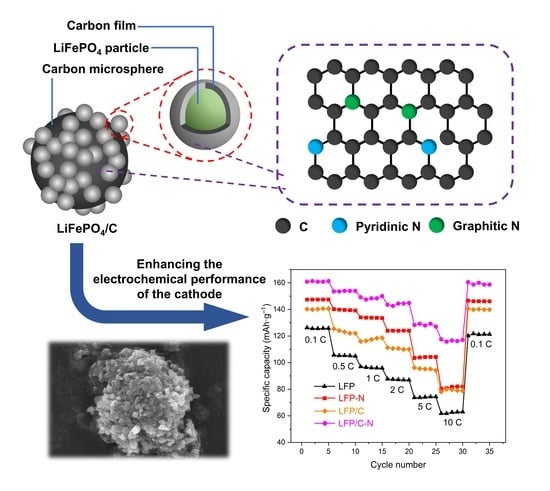
One-Pot Synthesis of LiFePO4/N-Doped C Composite Cathodes for Li-ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the LiFePO4/N-Doped C Composites
2.2. Structure and Morphology Characterizations
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Analysis of Structure and Morphology
3.2. Electrochemical Properties
| Coating Materials | Preparation Method | N Source | Discharge Capacity (mAh·g−1) | Ref. |
|---|---|---|---|---|
| N-doped C | High-temperature, solid-state method and subsequent calcination with addition of N and C sources | Ionic liquid1-butyl-3-methylimidazolium dicyanamide | 127.1 mAh·g−1 at 0.1 C | [47] |
| N-doped 3D graphene | Hydrothermal synthesis based on prepared N-doped graphene | Melamine | 125 mAh·g−1 at 5 C | [48] |
| N-doped C and TiO2 | Coating commercial LiFePO4 with TiO2 and C sources by wet chemical method | Polydopamine | 124 mAh·g−1 at 2 C | [49] |
| N-doped C | Solvothermal synthesis of LiFePO4 powder and subsequent calcination with addition of N and C sources | N-methyl-N-propylpyrrolidinium bis(trifluoromethyl sulfonyl)imide | 102.8 mAh·g−1 at 5 C | [50] |
| N-doped C | One-pot solvothermal method | CTAB | 128.4 mAh·g−1 at 5 C | This work |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Croguennec, L.; Palacin, M.R. Recent achievements on inorganic electrode materials for lithium-ion batteries. J. Am. Chem. Soc. 2015, 137, 3140–3156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Seh, Z.W.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Kwok, C.Y.; Nazar, L.F. Advances in lithium-sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 2016, 1, 16132. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Goodenough, Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Yang, J.; Li, Z.; Guang, T.; Hu, M.; Cheng, R.; Wang, R.; Shi, C.; Chen, J.; Hou, P.; Zhu, K.; et al. Green synthesis of high-performance LiFePO4 nanocrystals in pure water. Green Chem. 2018, 20, 5215–5223. [Google Scholar] [CrossRef]
- Yuan, L.-X.; Wang, Z.-H.; Zhang, W.-X.; Hu, X.-L.; Chen, J.-T.; Huang, Y.-H.; Goodenough, J.B. Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ. Sci. 2010, 4, 269–284. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Y.-J.; Luo, G.-Y.; Chen, Z.-L.; Wu, F.-Z.; Dai, X.-Y.; Mai, Y.; Li, J.-Q. Ni-doped LiFePO4/C as high-performance cathode composites for Li-ion batteries. Ceram. Int. 2020, 46, 14857–14863. [Google Scholar] [CrossRef]
- Zeng, J.; Wang, Q.; Wu, H.; Wu, J.; Jing, P.; Wang, Y.; Jiang, H.; Wei, Y.; Liu, H.; Zhang, Y. LiFePO4/carbon hybrids with fast Li-ion solid transfer capability obtained by adjusting the superheat temperature. J. Alloys Compd. 2019, 803, 998–1004. [Google Scholar] [CrossRef]
- Slawinski, W.A.; Playford, H.Y.; Hull, S.; Norberg, S.T.; Eriksson, S.G.; Gustafsson, T.; Edstrom, K.; Brant, W.R. Neutron Pair Distribution Function Study of FePO4 and LiFePO4. Chem. Mater. 2019, 31, 5024–5034. [Google Scholar] [CrossRef]
- Wei, X.; Guan, Y.; Zheng, X.; Zhu, Q.; Shen, J.; Qiao, N.; Zhou, S.; Xu, B. Improvement on high rate performance of LiFePO4 cathodes using graphene as a conductive agent. Appl. Surf. Sci. 2018, 440, 748–754. [Google Scholar] [CrossRef]
- Abdellahi, A.; Akyildiz, O.; Malik, R.; Thornton, K.; Ceder, G. Particle-size and morphology dependence of the preferred interface orientation in LiFePO4 nano-particles. J. Mater. Chem. A 2014, 2, 15437–15447. [Google Scholar] [CrossRef]
- Liu, H.C.; Wang, Y.M.; Hsieh, C.C. Optimized synthesis of Cu-doped LiFePO4/C cathode material by an ethylene glycol assisted co-precipitation method. Ceram. Int. 2017, 43, 3196–3201. [Google Scholar] [CrossRef]
- Lv, Y.J.; Huang, B.; Tan, J.X.; Jiang, S.Q.; Zhang, S.F.; Wen, Y.X. Enhanced low temperature electrochemical performances of LiFePO4/C by V3+ and F− co-doping. Mater. Lett. 2018, 229, 349–352. [Google Scholar] [CrossRef]
- Fan, C.L.; Lin, C.R.; Han, S.C.; Chen, J.; Li, L.F.; Bai, Y.M.; Zhang, K.H.; Zhang, X. Structure, conductive mechanism and electrochemical performances of LiFePO4/C doped with Mg2+, Cr3+ and Ti4+ by a carbothermal reduction method. N. J. Chem. 2014, 38, 795–801. [Google Scholar] [CrossRef]
- Hongtong, R.; Thanwisai, P.; Yensano, R.; Nash, J.; Srilomsak, S.; Meethong, N. Core-shell electrospun and doped LiFePO4/FeS/C composite fibers for Li-ion batteries. J. Alloys Compd. 2019, 804, 339–347. [Google Scholar] [CrossRef]
- Chang, Y.C.; Peng, C.T.; Hung, I.M. Effects of particle size and carbon coating on electrochemical properties of LiFePO4/C prepared by hydrothermal method. J. Mater. Sci. 2014, 49, 6907–6916. [Google Scholar] [CrossRef]
- Cogswell, D.A.; Bazant, M.Z. Size-dependent phase morphologies in LiFePO4 battery particles. Electrochem. Commun. 2018, 95, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Li, B.; Du, H.; Xu, C.; Kang, F. Effects of tin doping on physicochemical and electrochemical performances of LiFe1-xSnxPO4/C (0 <= x <= 0.07) composite cathode materials. Electrochim. Acta 2011, 56, 7385–7391. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Cheng, L.F.; Liu, Y.; Li, W.X.; Hu, P.F.; Jin, H.M.; Hu, Y.M.; Li, Y. LiFePO4/(C+Cu) composite with excellent cycling stability as lithium ion battery cathodes synthesized via a modified carbothermal reduction method. Ceram. Int. 2018, 44, 12106–12111. [Google Scholar] [CrossRef]
- Song, G.-M.; Wu, Y.; Xu, Q.; Liu, G. Enhanced electrochemical properties of LiFePO4 cathode for Li-ion batteries with amorphous NiP coating. J. Power Sources 2010, 195, 3913–3917. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Han, J.-M.; Myung, S.-T.; Lee, S.-W.; Amine, K. Significant improvement of high voltage cycling behavior AlF3-coated LiCoO2 cathode. Electrochem. Commun. 2006, 8, 821–826. [Google Scholar] [CrossRef]
- Dinh, H.-C.; Lim, H.; Park, K.D.; Yeo, I.-H.; Kang, Y.; Mho, S.-I. Long-term cycle stability at a high current for nanocrystalline LiFePO4 coated with a conductive polymer. Adv. Nat. Sci.-Nanosci. Nanotechnol. 2013, 4, 5. [Google Scholar] [CrossRef]
- Dinh, H.-C.; Mho, S.-I.; Yeo, I.-H. Electrochemical analysis of conductive polymer-coated LiFePO4 nanocrystalline cathodes with controlled morphology. Electroanalysis 2011, 23, 2079–2086. [Google Scholar] [CrossRef]
- O’Meara, C.; Karushev, M.; Polozhentceva, I.A.; Dharmasena, S.; Cho, H.; Yurkovich, B.J.; Kogan, S.; Kim, J.-H. Nickel-salen-type polymer as conducting agent and binder for carbon-free cathodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 2019, 11, 525–533. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, M.L.; Qian, J.; Ma, Y.T.; Li, Y.; Li, W.L.; Wang, F.J.; Li, L.; Wu, F.; Chen, R.J. Freestanding N-doped carbon coated CuO array anode for lithium-ion and sodium-ion batteries. Energy Technol. 2019, 7, 6. [Google Scholar] [CrossRef]
- Xue, H.; Yue, S.; Wang, J.; Zhao, Y.; Li, Q.; Yin, M.; Wang, S.; Feng, C.; Wu, Q.; Li, H.; et al. MoS2 microsphere@N-doped carbon composites as high performance anode materials for lithium-ion batteries. J. Electroanal. Chem. 2019, 840, 230–236. [Google Scholar] [CrossRef]
- Suo, G.; Ahmed, S.M.; Cheng, Y.; Zhang, J.; Li, Z.; Hou, X.; Yang, Y.; Ye, X.; Feng, L.; Zhang, L.; et al. Heterostructured CoS2/CuCo2S4@N-doped carbon hollow sphere for potassium-ion batteries. J. Colloid Interface Sci. 2022, 608, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Li, Y.; Xia, J.; Hu, Q.; Ke, X.; Ren, G.; Zhu, F. F-doped LiFePO4@N/B/F-doped carbon as high performance cathode materials for Li-ion batteries. Appl. Surf. Sci. 2019, 476, 761–768. [Google Scholar] [CrossRef]
- Zhang, B.F.; Xu, Y.L.; Wang, J.; Lin, J.; Wang, C.; Chen, Y.J. Lanthanum and cerium Co-doped LiFePO4: Morphology, electrochemical performance and kinetic study from−30–+50 degrees C. Electrochim. Acta 2019, 322, 134686. [Google Scholar] [CrossRef]
- Malik, R.; Burch, D.; Bazant, M.; Ceder, G. Particle size dependence of the ionic diffusivity. Nano Lett. 2010, 10, 4123–4127. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.A.J.; Islam, M.S. Surface structures and crystal morphologies of LiFePO4: Relevance to electrochemical behaviour. J. Mater. Chem. 2008, 18, 1209–1215. [Google Scholar] [CrossRef]
- Ye, N.; Yan, T.; Jiang, Z.; Wu, W.; Fang, T. A review: Conventional and supercritical hydro/solvothermal synthesis of ultrafine particles as cathode in lithium battery. Ceram. Int. 2018, 44, 4521–4537. [Google Scholar] [CrossRef]
- Wang, F.; Richards, V.N.; Shields, S.P.; Buhro, W.E. Kinetics and mechanisms of aggregative nanocrystal growth. Chem. Mater. 2013, 26, 5–21. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.; Seta, F.T.; Ma, M.; Shen, M.; Dai, L.; Liu, H.; Ni, Y. Improving dispersion stability of hydrochloric acid hydrolyzed cellulose nano-crystals. Carbohydr. Polym. 2019, 222, 115037. [Google Scholar] [CrossRef]
- El-Sheikh, S.; El-Sherbiny, S.; Barhoum, A.; Deng, Y. Effects of cationic surfactant during the precipitation of calcium carbonate nano-particles on their size, morphology, and other characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2013, 422, 44–49. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, H.; Xu, G.; Tan, Y.; Zhang, J.; Lv, X. Influence of CTAB and SDS on the properties of oil-in-water nano-emulsion with paraffin and span 20/Tween 20. Colloids Surf. A Physicochem. Eng. Asp. 2013, 418, 60–67. [Google Scholar] [CrossRef]
- Mao, Y.; Liao, J.; Wu, M.; Wen, J.; Xu, J.; Li, Y.; Xie, Y.; Ying, Q. Preparation of nano spherical bioglass by alkali-catalyzed mixed template. Mater. Res. Express 2020, 7, 105202. [Google Scholar] [CrossRef]
- Gao, C.; Zhou, J.; Liu, G.Z.; Wang, L. Synthesis of F-doped LiFePO4/C cathode materials for high performance lithium-ion batteries using co-precipitation method with hydrofluoric acid source. J. Alloys Compd. 2017, 727, 501–513. [Google Scholar] [CrossRef]
- Singh, I.; Kaur, G.; Bedi, R. CTAB assisted growth and characterization of nanocrystalline CuO films by ultrasonic spray pyrolysis technique. Appl. Surf. Sci. 2011, 257, 9546–9554. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, H.; Chen, K.; Lu, X.; Ouyang, P.; Fu, J. Enhancement in the aromatic yield from the catalytic fast pyrolysis of rice straw over hexadecyl trimethyl ammonium bromide modified hierarchical HZSM-5. Bioresour. Technol. 2018, 256, 241–246. [Google Scholar] [CrossRef]
- Sheng, Z.-H.; Shao, L.; Chen, J.-J.; Bao, W.-J.; Wang, F.-B.; Xia, X.-H. Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis. ACS Nano 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Fu, J.; Wang, K.; Liu, D.; Zhang, Z.; Sui, M.; Yan, P. b-Axis phase boundary movement induced (020) plane cracking in LiFePO4. ACS Appl. Mater. Interfaces 2020, 12, 39245–39251. [Google Scholar] [CrossRef]
- Meng, Y.; Han, W.; Zhang, Z.; Zhu, F.; Zhang, Y.; Wang, D. LiFePO4 particles coated with N-doped carbon membrane. J. Nanosci. Nanotechnol. 2017, 17, 2000–2005. [Google Scholar] [CrossRef]
- Luo, G.-Y.; Gu, Y.-J.; Liu, Y.; Chen, Z.-L.; Huo, Y.-L.; Wu, F.-Z.; Mai, Y.; Dai, X.-Y.; Deng, Y. Electrochemical performance of in situ LiFePO4 modified by N-doped graphene for Li-ion batteries. Ceram. Int. 2021, 47, 11332–11339. [Google Scholar] [CrossRef]
- Shi, J.Y.; Zhang, X.Q.; Zhang, X.K.; Xiang, Y. Titania and nitrogen-doped carbon co-modification: Their synergic effects on the electrochemical performance of LiFePO4. J. Alloys Compd. 2018, 750, 139–146. [Google Scholar] [CrossRef]
- Lv, C.; Duan, X.; Deng, J.; Wang, T. LiFePO4 mesocrystals coated with N-doped carbon from an ionic liquid for Li-ion batteries. CrystEngComm 2017, 19, 1253–1257. [Google Scholar] [CrossRef]
- Qiao, S.; Zhu, L.; Han, E.; Li, L.; Du, C.; He, Y. Synthesis and electrochemical properties of Na and Mg co-doped LiFe0.65Mn0.35PO4/C cathode materials for lithium-ion batteries. Int. J. Electrochem. Sci. 2019, 14, 11616–11629. [Google Scholar] [CrossRef]
- Li, L.; Tang, X.; Liu, H.; Qu, Y.; Lu, Z. Morphological solution for enhancement of electrochemical kinetic performance of LiFePO4. Electrochim. Acta 2010, 56, 995–999. [Google Scholar] [CrossRef]
- Tang, K.; Yu, X.; Sun, J.; Li, H.; Huang, X. Kinetic analysis on LiFePO4 thin films by CV, GITT, and EIS. Electrochim. Acta 2011, 56, 4869–4875. [Google Scholar] [CrossRef]








| Sample Name | Additives in Synthesis (mol·L−1) | Carbon Content (%) | Ohmic Resistance (Rs, Ω) | Charge Transfer Resistance (Rct, Ω) | Li+ Diffusion Coefficient (D, cm2 s−1) | |
|---|---|---|---|---|---|---|
| Glucose | CTAB | |||||
| LFP | 0 | 0 | 4.71 | 1.32 | 269 | 7.28 × 10−14 |
| LFP-N | 0 | 0.1 | 6.71 | 1.29 | 438 | 5.95 × 10−14 |
| LFP/C | 0.3 | 0 | 18.46 | 1.84 | 137 | 4.90 × 10−14 |
| LFP/C-N | 0.3 | 0.1 | 16.85 | 2.09 | 83.1 | 6.35 × 10−14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Wang, S.; Liu, L.; Li, Y.; Yang, J. One-Pot Synthesis of LiFePO4/N-Doped C Composite Cathodes for Li-ion Batteries. Materials 2022, 15, 4738. https://doi.org/10.3390/ma15144738
Zhang B, Wang S, Liu L, Li Y, Yang J. One-Pot Synthesis of LiFePO4/N-Doped C Composite Cathodes for Li-ion Batteries. Materials. 2022; 15(14):4738. https://doi.org/10.3390/ma15144738
Chicago/Turabian StyleZhang, Baoquan, Shuzhong Wang, Lu Liu, Yanhui Li, and Jianqiao Yang. 2022. "One-Pot Synthesis of LiFePO4/N-Doped C Composite Cathodes for Li-ion Batteries" Materials 15, no. 14: 4738. https://doi.org/10.3390/ma15144738
APA StyleZhang, B., Wang, S., Liu, L., Li, Y., & Yang, J. (2022). One-Pot Synthesis of LiFePO4/N-Doped C Composite Cathodes for Li-ion Batteries. Materials, 15(14), 4738. https://doi.org/10.3390/ma15144738