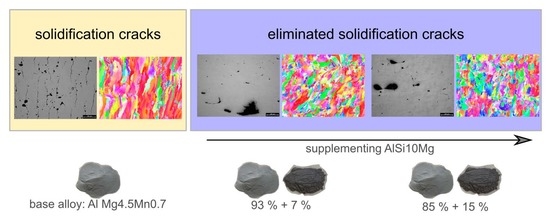
Practical Approach to Eliminate Solidification Cracks by Supplementing AlMg4.5Mn0.7 with AlSi10Mg Powder in Laser Powder Bed Fusion
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Metallographic Analysis of EN AW-5083 (AlMg4.5Mn0.7)
3.2. Modifying EN AW-5083 (AlMg4.5Mn0.7) with AlSi10Mg
3.2.1. Modification I: AlMg4.5Mn0.7 +7 wt.% AlSi10Mg
3.2.2. Modification II: AlMg4.5Mn0.7 +15 wt.% AlSi10Mg
3.3. Microstructure Analysis
3.3.1. EBSD Measurement
3.3.2. Qualitative EDX Measurement
4. Discussion
4.1. Mechanisms to Reduce Solidification Cracks
4.2. Feasibility of the Mixing Strategy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leirmo, J.L. High Strength Aluminium Alloys in Laser-Based Powder Bed Fusion—a Review. Procedia CIRP 2021, 104, 1747–1752. [Google Scholar] [CrossRef]
- Spierings, A.B. Dissertationsschrift Spierings. Available online: https://www.research-collection.ethz.ch/handle/20.500.11850/253924 (accessed on 9 January 2022).
- Aboulkhair, N.T.; Simonelli, M.; Parry, L.; Ashcroft, I.; Tuck, C.; Hague, R. 3D printing of Aluminium alloys: Additive Manufacturing of Aluminium alloys using selective laser melting. Prog. Mater. Sci. 2019, 106, 100578. [Google Scholar] [CrossRef]
- Ye, J.; Khairallah, S.A.; Rubenchik, A.M.; Crumb, M.F.; Guss, G.; Belak, J.; Matthews, M.J. Energy Coupling Mechanisms and Scaling Behavior Associated with Laser Powder Bed Fusion Additive Manufacturing. Adv. Eng. Mater. 2019, 21, 1900185. [Google Scholar] [CrossRef]
- Leis, A.; Weber, R.; Graf, T. Process Window for Highly Efficient Laser-Based Powder Bed Fusion of AlSi10Mg with Reduced Pore Formation. Materials 2021, 14, 5255. [Google Scholar] [CrossRef]
- Boley, C.D.; Khairallah, S.A.; Rubenchik, A.M. Calculation of laser absorption by metal powders in additive manufacturing. Appl. Opt. 2015, 54, 2477–2482. [Google Scholar] [CrossRef]
- Weingarten, C.; Buchbinder, D.; Pirch, N.; Meiners, W.; Wissenbach, K.; Poprawe, R. Formation and reduction of hydrogen porosity during selective laser melting of AlSi10Mg. J. Mater. Processing Technol. 2015, 221, 112–120. [Google Scholar] [CrossRef]
- Ewald, S.; Kies, F.; Hermsen, S.; Voshage, M.; Haase, C.; Schleifenbaum, J.H. Rapid Alloy Development of Extremely High-Alloyed Metals Using Powder Blends in Laser Powder Bed Fusion. Materials 2019, 12, 1706. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi-Tabasi, H.; Jhabvala, J.; Boillat, E.; Ivas, T.; Drissi-Daoudi, R.; Logé, R.E. An effective rule for translating optimal selective laser melting processing parameters from one material to another. Addit. Manuf. 2020, 36, 101496. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, C.; Escano, L.I.; Young, Z.; Xiong, L.; Fezzaa, K.; Everhart, W.; Brown, B.; Sun, T.; Chen, L. Transient dynamics of powder spattering in laser powder bed fusion additive manufacturing process revealed by in-situ high-speed high-energy x-ray imaging. Acta Mater. 2018, 151, 169–180. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.; Shi, Y.; Wang, L.; Jiang, W. Balling behavior of stainless steel and nickel powder during selective laser melting process. Int. J. Adv. Manuf. Technol. 2012, 59, 1025–1035. [Google Scholar] [CrossRef]
- Kaufmann, N.; Imran, M.; Wischeropp, T.M.; Emmelmann, C.; Siddique, S.; Walther, F. Influence of Process Parameters on the Quality of Aluminium Alloy EN AW 7075 Using Selective Laser Melting (SLM). Phys. Procedia 2016, 83, 918–926. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Hyer, H.; Park, S.; Pan, H.; Bai, Y.; Rice, K.P.; Sohn, Y. Microstructure and mechanical properties of Zr-modified aluminum alloy 5083 manufactured by laser powder bed fusion. Addit. Manuf. 2019, 28, 485–496. [Google Scholar] [CrossRef]
- Vock, S.; Klöden, B.; Kirchner, A.; Weißgärber, T.; Kieback, B. Powders for powder bed fusion: A review. Prog. Addit. Manuf. 2019, 4, 383–397. [Google Scholar] [CrossRef] [Green Version]
- Pleass, C.; Jothi, S. Influence of powder characteristics and additive manufacturing process parameters on the microstructure and mechanical behaviour of Inconel 625 fabricated by Selective Laser Melting. Addit. Manuf. 2018, 24, 419–431. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ishimoto, T.; Todo, T.; Wang, P.; Nakano, T. Influence of powder characteristics on densification via crystallographic texture formation: Pure tungsten prepared by laser powder bed fusion. Addit. Manuf. Lett. 2021, 1, 100016. [Google Scholar] [CrossRef]
- Khorasani, A.; Gibson, I.; Veetil, J.K.; Ghasemi, A.H. A review of technological improvements in laser-based powder bed fusion of metal printers. Int. J. Adv. Manuf. Technol. 2020, 108, 191–209. [Google Scholar] [CrossRef]
- Kou, S. Welding Metallurgy, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2003; ISBN 0471434914. [Google Scholar]
- Tang, Z.; Vollertsen, F. Influence of grain refinement on hot cracking in laser welding of aluminum. Weld. World 2014, 58, 355–366. [Google Scholar] [CrossRef]
- Schempp, P.; Rethmeier, M. Understanding grain refinement in aluminium welding: Henry Granjon Prize 2015 winner category B: Materials behaviour and weldability. Weld. World 2015, 59, 767–784. [Google Scholar] [CrossRef]
- Böhm, C.; Hagenlocher, C.; Wagner, J.; Graf, T.; Weihe, S. Analytical Description of the Criterion for the Columnar-To-Equiaxed Transition During Laser Beam Welding of Aluminum Alloys. Met. Mater. Trans. A 2021, 52, 2720–2731. [Google Scholar] [CrossRef]
- Weller, D.; Hagenlocher, C.; Weber, R.; Graf, T. Influence of the solidification path of AlMgSi aluminium alloys on the critical strain rate during remote laser beam welding. Sci. Technol. Weld. Join. 2020, 25, 101–105. [Google Scholar] [CrossRef]
- Hagenlocher, C.; Weller, D.; Weber, R.; Graf, T. Analytical Description of the Influence of the Welding Parameters on the Hot Cracking Susceptibility of Laser Beam Welds in Aluminum Alloys. Met. Mater. Trans. A 2019, 50, 5174–5180. [Google Scholar] [CrossRef]
- Montero-Sistiaga, M.L.; Mertens, R.; Vrancken, B.; Wang, X.; van Hooreweder, B.; Kruth, J.-P.; van Humbeeck, J. Changing the alloy composition of Al7075 for better processability by selective laser melting. J. Mater. Processing Technol. 2016, 238, 437–445. [Google Scholar] [CrossRef]
- Aversa, A.; Marchese, G.; Manfredi, D.; Lorusso, M.; Calignano, F.; Biamino, S.; Lombardi, M.; Fino, P.; Pavese, M. Laser Powder Bed Fusion of a High Strength Al-Si-Zn-Mg-Cu Alloy. Metals 2018, 8, 300. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.; Joguet, D.; Robin, G.; Peltier, L.; Laheurte, P. In situ elaboration of a binary Ti–26Nb alloy by selective laser melting of elemental titanium and niobium mixed powders. Mater. Sci. Eng. C 2016, 62, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Ewald, S.; Köhnen, P.; Ziegler, S.; Haase, C.; Schleifenbaum, J.H. Precise control of microstructure and mechanical properties of additively manufactured steels using elemental carbon powder. Mater. Lett. 2021, 295, 129788. [Google Scholar] [CrossRef]
- Shoji Aota, L.; Bajaj, P.; Zschommler Sandim, H.R.; Aimé Jägle, E. Laser Powder-Bed Fusion as an Alloy Development Tool: Parameter Selection for In-Situ Alloying Using Elemental Powders. Materials 2020, 13, 3922. [Google Scholar] [CrossRef]
- Bradford, R.L.; Cao, L.; Klosterman, D.; Herman, F.; Forman, L.; Browning, C. A metal–metal powder formulation approach for laser additive manufacturing of difficult-to-print high-strength aluminum alloys. Mater. Lett. 2021, 300, 130113. [Google Scholar] [CrossRef]
- Skelton, J.M.; Sullivan, E.J.; Fitz-Gerald, J.M.; Floro, J.A. Efficacy of elemental mixing of in situ alloyed Al-33wt%Cu during laser powder bed fusion. J. Mater. Processing Technol. 2022, 299, 117379. [Google Scholar] [CrossRef]
- Stopyra, W.; Gruber, K.; Smolina, I.; Kurzynowski, T.; Kuźnicka, B. Laser powder bed fusion of AA7075 alloy: Influence of process parameters on porosity and hot cracking. Addit. Manuf. 2020, 35, 101270. [Google Scholar] [CrossRef]
- Mehta, A.; Zhou, L.; Huynh, T.; Park, S.; Hyer, H.; Song, S.; Bai, Y.; Imholte, D.D.; Woolstenhulme, N.E.; Wachs, D.M.; et al. Additive manufacturing and mechanical properties of the dense and crack free Zr-modified aluminum alloy 6061 fabricated by the laser-powder bed fusion. Addit. Manuf. 2021, 41, 101966. [Google Scholar] [CrossRef]
- Nie, X.; Chen, Z.; Qi, Y.; Zhang, H.; Zhang, C.; Xiao, Z.; Zhu, H. Effect of defocusing distance on laser powder bed fusion of high strength Al–Cu–Mg–Mn alloy. Virtual Phys. Prototyp. 2020, 15, 325–339. [Google Scholar] [CrossRef]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Milkereit, B.; Yang, B.; Heiland, S.; Vieth, P.; Voigt, M.; Schaper, M.; Grundmeier, G.; Schick, C.; Kessler, O. Assessment of AlZnMgCu alloy powder modification for crack-free laser powder bed fusion by differential fast scanning calorimetry. Mater. Des. 2021, 204, 109677. [Google Scholar] [CrossRef]
- Heiland, S.; Milkereit, B.; Hoyer, K.-P.; Zhuravlev, E.; Kessler, O.; Schaper, M. Requirements for Processing High-Strength AlZnMgCu Alloys with PBF-LB/M to Achieve Crack-Free and Dense Parts. Materials 2021, 14, 7190. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, F. Anwendungstechnologie Aluminium; Springer Vieweg: Wiesbaden, Germany, 2014; ISBN 978-3-662-43806-0. [Google Scholar]
- Rappaz, M.; Drezet, J.-M.; Gremaud, M. A new hot-tearing criterion. Met. Mater. Trans. A 1999, 30, 449–455. [Google Scholar] [CrossRef]
- Kou, S. A criterion for cracking during solidification. Acta Mater. 2015, 88, 366–374. [Google Scholar] [CrossRef]
- Hirsch, J. Aluminium in Innovative Light-Weight Car Design. Mater. Trans. 2011, 52, 818–824. [Google Scholar] [CrossRef] [Green Version]
- DIN EN 573-3:2013-12: Aluminium and Aluminium Alloys—Chemical Composition and Form of Wrought Products—Part 3: Chemical Composition and Form of Products; German Version EN 573Y3; Beuth Publishing DIN: Berlin, Germany, 2013.
- Rasband, W.; Image, J. U.S. National Institutes of Health: Bethesda, MD, USA. Available online: http://imagej.nih.gov/ij2011 (accessed on 9 January 2022).
- Kouraytem, N.; Chiang, P.-J.; Jiang, R.; Kantzos, C.; Pauza, J.; Cunningham, R.; Wu, Z.; Tang, G.; Parab, N.; Zhao, C.; et al. Solidification crack propagation and morphology dependence on processing parameters in AA6061 from ultra-high-speed x-ray visualization. Addit. Manuf. 2021, 42, 101959. [Google Scholar] [CrossRef]
- Liu, J.; Kou, S. Susceptibility of ternary aluminum alloys to cracking during solidification. Acta Mater. 2017, 125, 513–523. [Google Scholar] [CrossRef] [Green Version]
- Bridgwater, J. Mixing of powders and granular materials by mechanical means—A perspective. Particuology 2012, 10, 397–427. [Google Scholar] [CrossRef]









| Si (wt.%) | Fe (wt.%) | Cu (wt.%) | Mn (wt.%) | Mg (wt.%) | Cr (wt.%) | Al (wt.%) | |
|---|---|---|---|---|---|---|---|
| EN AW-5083 (AlMg4.5Mn0.7) | 0.4 | 0.4 | 0.1 | 0.4–1.0 | 4.0–4.9 | 0.05–0.25 | Balance |
| Laser Power P (W) | Scan Velocity v (mm/s) | Focal Diameter df (µm) | Hatch Distance h (µm) | Volumetric Energy Density VED (J/mm3) | |
|---|---|---|---|---|---|
| A | 150 | 500 | 100 | 80 | 125 |
| B | 250 | 1000 | 100 | 80 | 104 |
| C | 250 | 1500 | 100 | 80 | 69 |
| D | 250 | 500 | 200 | 150 | 111 |
| E | 325 | 1000 | 200 | 150 | 72 |
| F | 350 | 1500 | 200 | 150 | 52 |
| Si (wt.%) | Mg (wt.%) | Mn (wt.%) | Al (wt.%) | Source | |
|---|---|---|---|---|---|
| EN AW-5083 (AlMg4.5Mn0.7) | 0.14 | 4.0 | 0.67 | Balance | Data sheet |
| AlSi10Mg | 9.84 | 0.32 | Balance | Data sheet | |
| MOD1: +7 wt.% AlSi10Mg | 0.82 | 3.74 | 0.62 | Balance | Calculated |
| MOD2: +15 wt.% AlSi10Mg | 1.60 | 3.45 | 0.57 | Balance | Calculated |
| Process Parameter | Density (%) |
|---|---|
| A | 97.49 |
| B | 96.03 |
| C | 96.92 |
| D | 97.39 |
| E | 96.88 |
| F | 95.44 |
| Process Parameter | Density (%) |
|---|---|
| A | 97.67 |
| B | 95.26 |
| C | 99.11 |
| D | 98.56 |
| E | 98.31 |
| F | 98.96 |
| Process Parameter | Density (%) |
|---|---|
| A | 98.51 |
| B | 96.60 |
| C | 98.61 |
| D | 99.10 |
| E | 98.89 |
| F | 97.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böhm, C.; Werz, M.; Weihe, S. Practical Approach to Eliminate Solidification Cracks by Supplementing AlMg4.5Mn0.7 with AlSi10Mg Powder in Laser Powder Bed Fusion. Materials 2022, 15, 572. https://doi.org/10.3390/ma15020572
Böhm C, Werz M, Weihe S. Practical Approach to Eliminate Solidification Cracks by Supplementing AlMg4.5Mn0.7 with AlSi10Mg Powder in Laser Powder Bed Fusion. Materials. 2022; 15(2):572. https://doi.org/10.3390/ma15020572
Chicago/Turabian StyleBöhm, Constantin, Martin Werz, and Stefan Weihe. 2022. "Practical Approach to Eliminate Solidification Cracks by Supplementing AlMg4.5Mn0.7 with AlSi10Mg Powder in Laser Powder Bed Fusion" Materials 15, no. 2: 572. https://doi.org/10.3390/ma15020572
APA StyleBöhm, C., Werz, M., & Weihe, S. (2022). Practical Approach to Eliminate Solidification Cracks by Supplementing AlMg4.5Mn0.7 with AlSi10Mg Powder in Laser Powder Bed Fusion. Materials, 15(2), 572. https://doi.org/10.3390/ma15020572