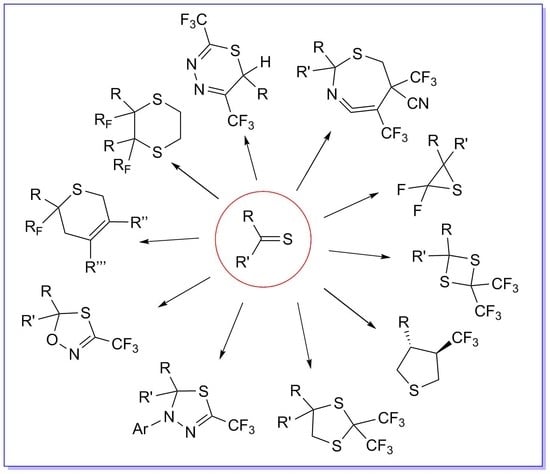
Synthesis of Fluorinated and Fluoroalkylated Heterocycles Containing at Least One Sulfur Atom via Cycloaddition Reactions †
Abstract
:1. Introduction
2. Three-Membered S-Heterocycles (Thiiranes)
3. Four-Membered S-Heterocycles
3.1. Thietanes
3.2. 1,3-Dithietanes
4. Five-Membered S-Heterocycles
4.1. Thiolanes (Tetrahydrothiophenes)
4.2. 1,3-Dithiolanes
4.3. 1,3-Dithioles
4.4. 1,2-Dithiolanes
4.5. 1,3-Oxathiolanes
4.6. 1,3,4-Thiadiazole Derivatives
4.7. 1,4,2-Oxathiazoles
5. Six-Membered S-Heterocycles
5.1. Thiopyran Derivatives
5.2. 1,4-Dithiines, 1,4-Dithianes and 1,2,4,5-Tetrathianes
5.3. 6H-1,3,4-Thiadiazines
6. Seven-Membered, Sulfur-Containing Heterocycles
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrov, V.A. (Ed.) Fluorinated Heterocyclic Compound: Synthesis, Chemistry, and Applications; J. Wiley & Sons, Inc: Hoboken, NJ, USA, 2009. [Google Scholar]
- Nenajdenko, V. (Ed.) Fluorine in Heterocyclic Chemistry, Vol. 1: 5-Membered Heterocycles and Macrocycles; Springer International Publishing: Manhattan, NY, USA, 2014. [Google Scholar]
- Luzzio, F.A. Synthesis and reactivity of fluorinated heterocycles. In Advances in Heterocyclic Chemistry; Academic Press: Cambridge, MA, USA, 2020; Volume 132, pp. 1–84. [Google Scholar]
- Mlostoń, G.; Romański, J.; Heimgartner, H. First synthesis of gem-difluorothiiranes from cycloaliphatic thioketones and difluorocarbene. Heterocycles 1999, 50, 403–410. [Google Scholar]
- Takayama, R.; Yamada, A.; Fuchibe, K.; Ichikawa, J. Synthesis of sulfanylated difluoroalkenes: Electrophilic difluoromethylidenation of dithioesters with difluorocarbene. Org. Lett. 2017, 19, 5050–5053. [Google Scholar] [CrossRef] [PubMed]
- Brasen, W.R.; Cripps, H.N.; Bottomley, C.G.; Farlow, M.W.; Krespan, C.G. Tetrafluorothiirane. J. Org. Chem. 1965, 30, 4188–4193. [Google Scholar] [CrossRef]
- Barney, A.L.; Bruce, J.M.; Coker, J.N.; Jacobson, H.W.; Sharkey, W.H. Fluorothiocarbonyl compounds. VI. Free-radical polymerization of thiocarbonyl fluoride Part A-1. J. Polymer Sci. 1966, 4, 2617–2636. [Google Scholar] [CrossRef]
- Diderrich, G.; Haas, A.; Yazdanbakhsch, M. Cyclization reactions of thiocarbonylhalogenides. Chem. Ber. 1977, 110, 916–920. [Google Scholar] [CrossRef]
- Middleton, M.J.; Sharkey, W.H. Fluorothiocarbonyl compounds. II. Reactions of hexafluorothioacetone. J. Org. Chem. 1965, 30, 1384–1390. [Google Scholar] [CrossRef]
- Mlostoń, G.; Gendek, T.; Heimgartner, H. Synthesis of trifluoromethyl-substituted sulfur heterocycles using 3,3,3-trifluoropyruvic acid derivatives. Helv. Chim. Acta 1996, 79, 1537–1548. [Google Scholar] [CrossRef]
- Rall, K.; Sundermeyer, W. Synthesis of perhalogenated 1,3-dithiethane S-oxides and thiiranes. J. Fluor. Chem. 1990, 47, 121–130. [Google Scholar] [CrossRef]
- Kowalski, M.K.; Obijalska, E.; Mlostoń, G.; Heimgartner, H. Generation and reactions of thiocarbonyl S-(2,2,2-trifluoroethanides). Synthesis of trifluoromethylated 1,3-dithiolanes, thiiranes and alkenes. J. Fluor. Chem. 2017, 200, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Middleton, W.J. Reactions of bis(trifluoromethyl)diazomethane with perfluorothiocarbonyl compounds. J. Org. Chem. 1969, 34, 3201–3202. [Google Scholar] [CrossRef]
- Mlostoń, G.; Heimgartner, H. Generation and typical reactions of thiocarbonyl ylides. Pol. J. Chem. 2000, 74, 1503–1533. [Google Scholar]
- Middleton, W.J. Fluorothiocarbonyl compounds. IV. Hexafluorothioacetone-Olefin adducts. J. Org. Chem. 1965, 30, 1395–1398. [Google Scholar] [CrossRef]
- Kitazume, T.; Otaka, T.; Takei, R.; Ishikawa, N. Preparation and reactions of 4-alkoxy-2,2-bis(trifluoromethyl)thietanes and 5-alkoxy-3,3-bis(trifluoromethyl)dithiolanes. Bull. Chem. Soc. Jpn. 1976, 49, 2491–2494. [Google Scholar] [CrossRef] [Green Version]
- Petrov, V.A.; Marshall, W. Remarkable effect of metal fluoride catalyst on reaction of hexafluoropropene, sulfur and vinyl ethers. Convenient synthesis of 2,2-bis(trifuoromethyl)-4-R-thietanes, 3,3-bis(trifluoromethyl)-5-R-1,2-dithiolanes and 2,2-bis(trifluoromethyl)-4-R-1,3-dithiolanes. J. Fluor. Chem. 2010, 131, 1144–1155. [Google Scholar]
- Petrov, V.A.; Marshall, W. Reaction of 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithiethane with N-vinyl compounds. Beilstein J. Org. Chem. 2013, 9, 2615–2619. [Google Scholar] [CrossRef] [Green Version]
- Petrov, V.A.; Marshall, W. Do reactions of 2,2,4,4-tetrakis(trifluoromethyl)-1,3-dithietane require fluoride anion catalysis? J. Fluor. Chem. 2015, 179, 56–63. [Google Scholar] [CrossRef]
- Petrov, V.A.; Marshall, W. Reaction of hexafluorothioacetone dimer with ketene dimethylacetal and dimethyl thioacetal. J. Fluor. Chem. 2012, 143, 220–225. [Google Scholar] [CrossRef]
- Petrov, V.A.; Krespan, C.G.; Marshall, W. Reaction of quadricyclane with fluorinated sulfur-containing compounds. J. Fluor. Chem. 2005, 126, 1332–1341. [Google Scholar] [CrossRef]
- Smart, B.E.; Middleton, W.J. Bis(trifluoromethyl)sulfene: Generation and cycloaddition reactions. J. Am. Chem. Soc. 1987, 109, 4982–4992. [Google Scholar] [CrossRef]
- Schuler, B.; Sundermeyer, W. Thermolysis of oxidized 1,3-dithiolanes: A new route for the synthesis of thiocabonyl compounds and sulfines. Chem. Ber. 1990, 123, 177–184. [Google Scholar] [CrossRef]
- Mykhaylychenko, S.S.; Pikun, N.V.; Shermolovich, Y.G. Acylation of primary polyfluoroalkanethioamides. J. Fluor. Chem. 2012, 140, 76–81. [Google Scholar] [CrossRef]
- Ogurok, V.M. 2,2,4,4-Tetrakis(trifluoromethyl)-1,3-dithietane in organic synthesis. Chem. Heterocycl. Comp. 2016, 52, 211–212. [Google Scholar] [CrossRef]
- Raasch, M.S. Bis(trifluoromethyl)thioketene. I. Synthesis and cycloaddition reactions. J. Org. Chem. 1970, 35, 3470–3483. [Google Scholar] [CrossRef]
- Mondal, B.; Nandi, S.; Pan, S.C. Organocatalytic asymmetric synthesis of tetrahydrothiophenes and tetrahydrothiopyrans. Eur. J. Org. Chem. 2017, 4666–4677. [Google Scholar] [CrossRef]
- Sato, H.; Yoshimura, Y.; Sakata, S.; Miura, S.; Machida, H.; Matsuda, A. Synthesis of L-enantiomers of 4′-thioarabinofuranosyl pyrimidine nucleosides. Bioorg. Med. Chem. Lett. 1998, 8, 989–992. [Google Scholar] [CrossRef]
- Volkmann, R.A.; Kelbaugh, P.R.; Nason, D.M.; Jasys, V.J. 2-Thioalkyl penems: An efficient synthesis of sulopenem, a (5R,6S)-6-(1(R)-hydroxyethyl)-2-[(cis-1-oxo-3-thiolanyl)thio-2-penem antibacterial. J. Org. Chem. 1992, 57, 4352–4361. [Google Scholar] [CrossRef]
- Mlostoń, G.; Grzelak, P.; Heimgartner, H. Strong influence of the trifluoromethyl group on the chemoselectivity of [3+2]-cycloadditions of thiocarbonyl S-methanides with α,β-unsaturated ketones. J. Fluor. Chem. 2016, 190, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Markitanov, Y.M.; Timoshenko, V.M.; Rudenko, T.V.; Rusanov, E.B.; Shermolovich, Y.G. 3-Functional substituted 4-trifluoromethyl tetrahydrothiophenes via [3+2]-cycloaddition reactions. J. Sulfur Chem. 2019, 40, 629–640. [Google Scholar] [CrossRef]
- Siry, S.A.; Timoshenko, V.M.; Vlasenko, Y.G.; Baranova, G.V.; Zagorodyna, S.D.; Nesterova, N.V. Pummerer reactions of thiopyran derivatives as a method for the preparation of trifluoromethyl-substituted thiolanes with antiviral activity. Chem. Heterocycl. Comp. 2014, 50, 467–478. [Google Scholar] [CrossRef]
- Mykhaylychenko, S.S.; Markitanov, Y.N.; Rudenko, T.V.; Rusanov, E.B.; Shermolovich, Y.G. Synthesis of 4-polyfluoroalkyl-1,3-dithiolanes via [3+2]-cycloaddition of thiocarbonyl ylide to polyfluoroalkanethioamides. Chem. Heterocycl. Comp. 2019, 55, 189–192. [Google Scholar] [CrossRef]
- Asr, A.; Emamian, S.; Aghaie, M.; Aghaie, H. [3+2]-Cycloaddition reaction between CF3-substituted thiocarbonyl ylides and thiketones: Exploration of regioselectivity and mechanistic aspects using molecular electron density theory. J. Fluor. Chem. 2018, 209, 14–22. [Google Scholar] [CrossRef]
- Mlostoń, G.; Urbaniak, K.; Robak, A.; Wręczycki, J.; Bielinski, D.; Weigand, W. Efficient sulfurization of 2,3-diarylcyclopropenthiones using tetrabutylammonium fluoride (TBAF) as a convenient source of the fluoride anion. J. Sulfur Chem. 2022. submitted. [Google Scholar]
- Wu, J.; Gao, W.-X.; Huang, X.-B.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Selective [3+2]-cycloaddition of cyclopropenone derivatives and elemental chalcogens. Org. Lett. 2020, 22, 5555–5560. [Google Scholar] [CrossRef] [PubMed]
- Sánchez García, J.J.; Joo-Cisneros, R.S.; García-Bassoco, D.; Flores-Alamo, M.; Méndez Stivalet, J.M.; García-Valdés, J. Synthesis, characterization, and oxidation electrochemistry of some novel 1,2-dithiol-3-ones and 1,2-dithiole-3-thiones containing aryl and metallocenyl fragments. J. Organometal. Chem. 2021, 944, 121809. [Google Scholar] [CrossRef]
- Rakitin, O.A. Synthesis and reactivity of 3H-1,2-dithiolane-3-thione. Molecules 2021, 26, 3595. [Google Scholar] [CrossRef]
- Mykhaylychenko, S.S.; Pikun, N.V.; Rusanov, E.B.; Rozhenko, A.B.; Shermolovich, Y.G. Synthesis of 2-polyfluoroalkyl-2,3-dihydro-1,3,4-thiadiazoles via regioselective [3+2]-cycloaddition of nitrile imines to polyfluoroalkanethioamides. Chem. Heterocycl. Comp. 2017, 53, 1268–1276. [Google Scholar] [CrossRef]
- Utecht-Jarzyńska, G.; Mykhaylychenko, S.S.; Rusanov, E.B.; Shermolovich, Y.G.; Jasińsky, M.; Mlostoń, G. Highly fluorinated 2,3-dihydro-1,3,4-thiadiazole derivatives via [3+2]-cycloadditions of tertiary thioamides with nitrile imines derived from trifluoroacetonitrile. J. Fluor. Chem. 2021, 242, 109702. [Google Scholar] [CrossRef]
- Mlostoń, G.; Urbaniak, K.; Utecht, G.; Lenz, D.; Jasińsky, M. Trifluoromethylated 2,3-dihydro-1,3,4-thiadiazoles via the regioselective [3+2]-cycloaddition of fluorinated nitrile imines with aryl, hetaryl, and ferrocenyl thioketones. J. Fluor. Chem. 2016, 192, 147–154. [Google Scholar] [CrossRef]
- Utecht, G.; Sioma, J.; Jasińsky, M.; Mlostoń, G. Expected and unexpected results in reactions of fluorinated nitrile imines with (cyclo)aliphatic thioketones. J. Fluor. Chem. 2017, 201, 68–75. [Google Scholar] [CrossRef]
- Utecht-Jarzyńska, G.; Jasińsky, M.; Świątek, K.; Mlostoń, G.; Heimgartner, H. Novel trifluoromethylated spiro-1,3,4-thiadiazoles via [3+2]-cycloadditions of 2,3-diphenylcyclopropenethione with selected in situ-generated nitrile imines derived from trifluoroacetonitrile. Heterocycles 2020, 101, 251–262. [Google Scholar]
- Grzelak, P.; Utecht, G.; Jasińsky, M.; Mlostoń, G. First (3+2)-cycloadditions of thiochalcones as C=S dipolarophiles: Efficient synthesis of 1,3,4-thiadiazoles via reactions with fluorinated nitrile imines. Synthesis 2017, 49, 2129–2137. [Google Scholar]
- Hassaneen, H.M.; Hilal, R.H.; Elwan, N.M.; Harhash, A.; Shawali, A.S. The regioselectivity in the formation of pyrazolines and pyrazoles from nitrile imines. J. Heterocycl. Chem. 1984, 21, 1013–1016. [Google Scholar] [CrossRef]
- Atir, R.; Mallouk, B.S.; Bougrin, K.; Soufiaoui, M.; Laghzizil, A. Porous calcium hydroxyapatite as an efficient catalyst for synthesis of pyrazolines via 1,3-dipolar cycloaddition under solvent-free microwave irradiation. Synth. Commun. 2006, 36, 111–120. [Google Scholar] [CrossRef]
- Huisgen, R.; Langhals, E.; Mlostoń, G.; Oshima, T. A stable seven-membered ring ketene imine from a thiocarbonyl ylide and an acceptor olefine. Heterocycles 1989, 29, 2069–2074. [Google Scholar] [CrossRef]
- Huisgen, R.; Langhals, E.; Nöth, H. A crystalline seven-membered cyclic ketene imine from a thiocarbonyl S-methylide. J. Org. Chem. 1990, 55, 1412–1414. [Google Scholar] [CrossRef]
- Huisgen, R.; Langhals, E.; Polborn, K.; Karaghiosoff, K. Cyclic seven-membered ketene imines from hindered thiocarbonyl ylides and 2,3-bis(trifluoromethyl)fumaronitrile: Properties and surprising reactions. Helv. Chim. Acta 2004, 87, 1426–1445. [Google Scholar] [CrossRef]
- Ni, C.; Hu, J. The unique fluorine effects in organic reactions: Recent facts and insights into fluoroalkylations. Chem. Soc. Rev. 2016, 45, 5441–5454. [Google Scholar] [CrossRef] [Green Version]
- Mlostoń, G.; Kowalski, M.K.; Obijalska, E.; Heimgartner, H. Efficient synthesis of fluoroalkylated 1,4,2-oxathiazoles via regioselective [3+2]-cycloaddition of fluorinated nitrile oxides with thioketones. J. Fluor. Chem. 2017, 199, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Poh, J.-S.; Garcia-Ruiz, C.; Zúñiga, A.; Meropni, F.; Blakemore, D.C.; Browne, D.L.; Ley, S.V. Synthesis of trifluoromethylated isoxazoles and their elaboration through inter- and intramolecular C–H arylation. Org. Biomol. Chem. 2016, 14, 5983–5991. [Google Scholar] [CrossRef] [Green Version]
- Vyas, D.M.; Hay, G.W. Studies on the synthesis of novel carbohydrates with sulfur in the ring. Part III. The synthesis of derivatives of 2,6-dithio-hex-4-en-2-ulopyranosidononitriles. Can. J. Chem. 1975, 53, 1362–1366. [Google Scholar] [CrossRef]
- Adam, D.; Freer, A.A.; Isaacs, N.W.; Kirby, G.M.; Littlejohn, A.; Rahman, M.S. Synthesis of a thiashikimic acid derivative. J. Chem. Soc. Perkin Trans. 1 1992, 261–1264. [Google Scholar] [CrossRef]
- Schuler, B.; Sundermeyer, W. Trifluorothioacetaldehyde. Tetrahedron Lett. 1989, 30, 4111–4112. [Google Scholar] [CrossRef]
- Markovskii, L.N.; Slyusarenko, E.I.; Shermolovich, Y.G. 7-Hydrododecafluoroheptanethial oxide. J. Org. Chem. USSR 1990, 26, 912–914. [Google Scholar]
- Ogurok, V.M.; Siry, S.A.; Rusanov, E.B.; Shermolovich, Y.G. Polyfluoroalkyl sulfines derived from 1,1-dihydropolyfluoroalkanesulfinyl chlorides: Decomposition and [4+2]-cycloaddition reactions. J. Fluor. Chem. 2016, 189, 119–126. [Google Scholar] [CrossRef]
- Markovskii, L.N.; Kornuta, P.P.; Shermolovich, Y.G. Reaction of polyfluorinated aliphatic thioaldehydes with 1-ethoxy-1,3-butadiene. Russ. J. Org. Chem. 1999, 35, 1143–1145. [Google Scholar]
- Rohr, U.; Schatz, J.; Sauer, J. Thio- and selenocarbonyl compounds as “superdienophiles” in [4+2] cycloadditions. Eur. J. Org. Chem. 1998, 2875–2883. [Google Scholar] [CrossRef]
- Breu, J.; Höcht, P.; Rohr, U.; Schatz, J.; Sauer, J. Thiocarbonyl compounds in [4+2] cycloadditions: Preparative aspects. Eur. J. Org. Chem. 1998, 2861–2873. [Google Scholar] [CrossRef]
- Petrov, V.; Marchione, A.A.; Dooley, R. Diels-Alder reactions of “in situ” generated perfluorinated thioketones. Chem. Commun. 2018, 54, 9298–9300. [Google Scholar] [CrossRef]
- Petrov, V.; Dooley, R.J.; Marchione, A.A.; Diaz, E.L.; Clem, B.S. Simple protocol for preparation of Diels-Alder adducts of perfluorinated thioketones. J. Fluor. Chem. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Petrov, V.A.; Marchione, A.A.; Dooley, R.; Marshall, W. Regiochemistry of Diels-Alder reaction of hexafluorothioacetone and dienes. J. Fluor. Chem. 2017, 196, 7–13. [Google Scholar] [CrossRef]
- Middleton, W.J.; Howard, E.G.; Sharkev, W.H. Perfluorothiocarbonyl compounds. J. Am. Chem. Soc. 1961, 83, 2589–2590. [Google Scholar] [CrossRef]
- Middleton, W.J. Fluorothiocarbonyl compounds. III. Diels-Alder reactions. J. Org. Chem. 1965, 30, 1390–1394. [Google Scholar] [CrossRef]
- Siry, S.A.; Timoshenko, V.M. 2-Polyfluoroalkyl thiopyrylium salts: Synthesis and reactions with nucleophiles. Tetrahedron Lett. 2010, 51, 6406–6409. [Google Scholar] [CrossRef]
- Siry, S.A.; Timoshenko, V.M.; Shermolovich, Y.G. Sulfur-assisted ring contraction of polyfluoroalkylthiopyran derivatives as a route to functionalized fluorine-containing thiophenes. J. Fluor. Chem. 2016, 181, 17–21. [Google Scholar] [CrossRef]
- Portella, C.; Shermolovich, Y.G.; Tschenn, O. Synthesis of alkyl perfluoroalkanedithiocarboxylates and some aspects of their reactivity in cycloaddition reactions. Bull. Soc. Chim. Fr. 1997, 134, 697–702. [Google Scholar]
- Laduron, F.; Nyns, C.; Janousek, Z.; Viehe, H.G. Synthesis and reactivity of trifluorodithioacetates derived from trifluorothioacetamides. J. Pract. Chem. 1997, 339, 697–707. [Google Scholar] [CrossRef]
- Timoshenko, V.M.; Siry, S.A.; Rozhenko, A.B.; Shermolovich, Y.G. Asymmetric induction in thia-Diels-Alder reactions of chiral polyfluoroalkylthionocarboxylates. J. Fluor. Chem. 2010, 131, 172–183. [Google Scholar] [CrossRef]
- Mikhailichenko, S.S.; Bouillon, J.-P.; Besson, T.; Shermolovich, Y.G. First synthesis of 2-aminosubstituted-2-perfluoroalkyl-3,6-dihydro-2H-thiopyrans by hetero-Diels-Alder reactions of fluorinated thioamides under microwave heating. Tetrahedron Lett. 2010, 51, 990–993. [Google Scholar] [CrossRef]
- Krespan, C.G.; McKusick, B.C. Bis-(polyfluoroalkyl)-acetylenes. V. Addition of bis-(trifluoromethyl)-1,2-dithietene to olefins and acetylenes. J. Am. Chem. Soc. 1961, 83, 3438–3440. [Google Scholar] [CrossRef]
- Mlostoń, G.; Romański, J.; Rusanov, E.B.; Chernega, A.N.; Shermolovich, Y.G. Reaction of benzyl ω-H-perfluorodithiovalerate with diazomethane. Russ. J. Org. Chem. 1995, 31, 952–955. [Google Scholar]
- Elsäßer, A.; Sundermeyer, W. Bis(trifluoromethyl)sulfine, (CF3)2C=SO: New syntheses and some reactions. Chem. Ber. 1985, 118, 4553–4560. [Google Scholar] [CrossRef]
- Seitz, G.; Mohr, R.; Overheu, W.; Allmann, R.; Nagel, M. Donor-substituted thiocarbonyl functions as heterodienophiles in [4+2]-cycloadditions with inverse electron demand. Angew. Chem. Int. Ed. Engl. 1984, 23, 890–891. [Google Scholar] [CrossRef]
- Huisgen, R.; Mlostoń, G.; Langhals, E.; Oshima, T. Reactions of sterically hindered ‘thiocarbonyl ylides’ with 1,2-bis(trifluoromethyl)ethene-1,2-dicarbonitrile: Isolation of a seven membered ketene imine. Helv. Chim. Acta 2002, 85, 2668–2685. [Google Scholar] [CrossRef]
- Giera, H.; Huisgen, R.; Polborn, K. Cycloadditions with cyclic seven-membered ketene imines. Eur. J. Org. Chem. 2005, 2005, 3781–3790. [Google Scholar] [CrossRef]
- Langhals, E.; Huisgen, R.; Polborn, K. A novel dimerization mode of a cyclic ketene imine. Chem. Eur. J. 2004, 10, 4353–4357. [Google Scholar] [CrossRef]
- Okazaki, R.; Inoue, K.; Inamoto, N. Reactions of ketone hydrazones and ß-keto enamines with disulfur dichloride. New synthesis of thioketones and 5H-1,2,3-dithiazoles. Bull. Chem. Soc. Jpn. 1981, 54, 3541–3545. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yan, K.; Fu, C.; Peng, H.; Hawker, C.J.; Andrew, K.; Whittaker, A.K. Biological utility of fluorinated compounds: From materials design to molecular omaging, Therapeutics and environmental remediation. Chem. Rev. 2022, 122, 167–208. [Google Scholar] [CrossRef]
- Naumenko, K.; Golovan, A.; Zagorognya, S.; Nesteroba, N.; Siryy, S.; Markitanov, Y.; Tymoshenko, V.; Shermolovytch, Y. Cytotoxicity and antiviral activity of novel fluorine compounds. Bull. Taras Shevchenko Natl. Univ. Kyiv 2013, 65, 82–86. [Google Scholar]
- Al-Harthy, T.; Zoghaib, W.; Abdel-Jalil, R. Importance of fluorine in benzazole compounds. Molecules 2020, 25, 4677. [Google Scholar] [CrossRef]
- Serdyuk, O.V.; Abaev, V.T.; Butin, A.V.; Nenajdenko, V.G. Synthesis of fluorinated thiophenes and their analogues. Synthesis 2011, 2505–2529. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, L.; Jiao, X.; Peng, Z.; Liu, S.; Rech, J.J.; Klump, E.; Ade, H.; So, F.; You, W. Fluorinated thiophene units improve photovoltaic device performance of donor–acceptor copolymers. Chem. Mater. 2017, 29, 5990–6002. [Google Scholar] [CrossRef]
- Thomson, N.; Kanibolotsky, A.L.; Cameron, J.; Tuttle, T.; Findlay, N.J.; Skabara, P.J. Incorporation of perfluorohexyl-functionalised thiophenes into oligofluorene-truxenes: Synthesis and physical properties. Beilstein J. Org. Chem. 2013, 9, 1243–1251. [Google Scholar] [CrossRef]
- Roy, C.; Bura, T.; Beaupré, S.; Légaré, M.-A.; Sun, J.-P.; Hill, I.G.; Leclerc, M. Fluorinated thiophene-based synthons: Polymerization of 1,4-dialkoxybenzene and fluorinated dithieno-2,1,3-benzothiadiazole by direct heteroarylation. Macromolecules 2017, 50, 4658–4667. [Google Scholar] [CrossRef]
- Ni, C.; Hu, M.; Hu, J. Good partnership between sulfur and fluorine: Sulfur-based fluorination and fluoroalkylation reagents for organic synthesis. Chem. Rev. 2015, 115, 765–825. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R.; Li, X.; Giera, H.; Langhals, E. ‘Thiobenzophenone S-methylide’ (= (Diphenylmethylidenesulfonio)methanide), and C,C multiple bonds: Cycloadditions and dipolarophilic reactivities. Helv. Chim. Acta 2001, 84, 981–999. [Google Scholar] [CrossRef]
- Dawid, M.; Mlostoń, G.; Warkentin, J. Relative reactivities of carbonyl and thiocarbonyl groups toward dimethoxycarbene: Two new dimethoxythiiranes. Chem. Eur. J. 2002, 8, 2184–2187. [Google Scholar] [CrossRef]
- Mlostoń, G.; Kula, K.; Jasiński, R. A DFT study on the molecular mechanism of additions of electrophilic and nucleophilic carbenes to non-enolizable cycloaliphatic thioketones. Molecules 2021, 26, 5562. [Google Scholar] [CrossRef]
- Kondratov, I.S.; Tolmachova, N.A.; Haufe, G. Diels–Alder reaction in the synthesis of fluorinated (hetero)aromatic compounds. Eur. J. Org. Chem. 2018, 3618–3647. [Google Scholar] [CrossRef]
- Blond, G.; Gulea, M.; Mamane, V. Recent contributions to hetero Diels-Alder reactions. Curr. Org. Chem. 2016, 20, 2161–2210. [Google Scholar] [CrossRef]






































| Products | R1 | R2 | R3 | R4 | Yield (%) |
|---|---|---|---|---|---|
| 2h | CF3 | CF3 | Ph | Ph | 77.5 [9] |
| 2i | Ph | Ph | CF3 | CO2Me | 57 [10] |
| 2j | Cl | Cl | CF3 | CF3 | 49 [11] |
| 2k | F | Cl | CF3 | CF3 | 60 [11] |
| 2l | CF3 | H |  | 81 [12] | |
| 6a | CF3 | H | 79 [12] | ||
| 2m | CF3 | H |  | 75 [12] | |
| 6b | CF3 | H | 71 [12] | ||
| 6c | CF3 | CF3 | CF3 | CF3 | 87 [13] |
| RF | Cycloadduct 73 | Reaction Conditions (Excess of Diene) | Yield (%) | Diastereomeric Excess (de) (%) | |
|---|---|---|---|---|---|
| a | CF3 H(CF2)2 |  | −20 °C–20 °C 1–4 days | 75–83 | 14–16 |
| b | CF3 H(CF2)2 |  | −20 °C–20 °C 1–6 days | 90–94 | 50–60 |
| c | CF3 |  | 20 °C 3 days | 90 | 56 |
| d | CF3 H(CF2)2 |  | −20 °C–20 °C 3–6 days | 85–98 | 16–20 |
| e | CF3 H(CF2)2 |  | 130 °C 5 h | 75–80 | 6–20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlostoń, G.; Shermolovich, Y.; Heimgartner, H. Synthesis of Fluorinated and Fluoroalkylated Heterocycles Containing at Least One Sulfur Atom via Cycloaddition Reactions. Materials 2022, 15, 7244. https://doi.org/10.3390/ma15207244
Mlostoń G, Shermolovich Y, Heimgartner H. Synthesis of Fluorinated and Fluoroalkylated Heterocycles Containing at Least One Sulfur Atom via Cycloaddition Reactions. Materials. 2022; 15(20):7244. https://doi.org/10.3390/ma15207244
Chicago/Turabian StyleMlostoń, Grzegorz, Yuriy Shermolovich, and Heinz Heimgartner. 2022. "Synthesis of Fluorinated and Fluoroalkylated Heterocycles Containing at Least One Sulfur Atom via Cycloaddition Reactions" Materials 15, no. 20: 7244. https://doi.org/10.3390/ma15207244
APA StyleMlostoń, G., Shermolovich, Y., & Heimgartner, H. (2022). Synthesis of Fluorinated and Fluoroalkylated Heterocycles Containing at Least One Sulfur Atom via Cycloaddition Reactions. Materials, 15(20), 7244. https://doi.org/10.3390/ma15207244