Ionic Liquid Mixture Electrolyte Matching Porous Carbon Electrodes for Supercapacitors
Abstract
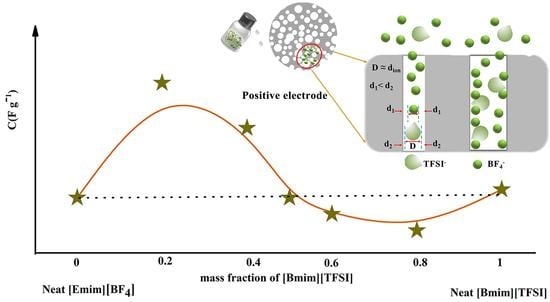
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Binary IL Mixture Electrolytes
2.3. Characterization
2.4. Preparation of Electrodes and Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yang, L.; Zhuo, K.; Xu, X.; Zhang, Z.; Du, Q.; Chen, Y.; Sun, D.; Wang, J. Redox-active phthalocyanine-decorated graphene aerogels for high-performance supercapacitors based on ionic liquid electrolyte. J. Mater. Chem. A 2020, 8, 21789–21796. [Google Scholar] [CrossRef]
- Xue, Q.; Gan, H.; Huang, Y.; Zhu, M.; Pei, Z.; Li, H.; Deng, S.; Liu, F.; Zhi, C. Boron Element Nanowires Electrode for Supercapacitors. Adv. Energy Mater. 2018, 8, 1703117. [Google Scholar] [CrossRef]
- Lindberg, S.; Cavallo, C.; Calcagno, G.; Navarro-Suárez, A.M.; Johansson, P.; Matic, A. Electrochemical Behaviour of Nb-Doped Anatase TiO2 Microbeads in an Ionic Liquid Electrolyte. Batter. Supercaps 2020, 3, 1233–1238. [Google Scholar] [CrossRef]
- Hussain, S.; Amade, R.; Boyd, A.; Musheghyan-Avetisyan, A.; Alshaikh, I.; Martí-Gonzalez, J.; Pascual, E.; Meenan, B.J.; Bertran-Serra, E. Three-dimensional Si/vertically oriented graphene nanowalls composite for supercapacitor applications. Ceram. Int. 2021, 47, 21751–21758. [Google Scholar] [CrossRef]
- Ubaidullah, M.; Ahmed, J.; Ahamad, T.; Shaikh, S.F.; Alshehri, S.M.; Al-Enizi, A.M. Hydrothermal synthesis of novel nickel oxide@nitrogenous mesoporous carbon nanocomposite using costless smoked cigarette filter for high performance supercapacitor. Mater. Lett. 2020, 266, 127492. [Google Scholar] [CrossRef]
- Ubaidullah, M.; Al-Enizi, A.M.; Ahamad, T.; Shaikh, S.F.; Al-Abdrabalnabi, M.A.; Samdani, M.S.; Kumar, D.; Alam, M.A.; Khan, M. Fabrication of highly porous N-doped mesoporous carbon using waste polyethylene terephthalate bottle-based MOF-5 for high performance supercapacitor. J. Energy Storage 2021, 33, 102125. [Google Scholar] [CrossRef]
- Tiwari, N.; Kadam, S.; Ingole, R.; Kulkarni, S. Facile hydrothermal synthesis of ZnFe2O4 nanostructures for high-performance supercapacitor application. Ceram. Inter. 2022, 48, 29478–29483. [Google Scholar] [CrossRef]
- Du, Q.; Zhao, Y.; Zhuo, K.; Chen, Y.; Yang, L.; Wang, C.; Wang, J. 3D hierarchical porous carbon matching ionic liquid with ultrahigh specific surface area and appropriate porous distribution for supercapacitors. Nanoscale 2021, 13, 13285–13293. [Google Scholar] [CrossRef]
- Huang, P.-L.; Luo, X.-F.; Peng, Y.-Y.; Pu, N.-W.; Ger, M.-D.; Yang, C.-H.; Wu, T.-Y.; Chang, J.-K. Ionic Liquid Electrolytes with Various Constituent Ions for Graphene-based Supercapacitors. Electrochim. Acta 2015, 161, 371–377. [Google Scholar] [CrossRef]
- Yadav, N.; Hashmi, S.A. Hierarchical porous carbon derived from eucalyptus-bark as a sustainable electrode for high-performance solid-state supercapacitors. Sustain. Energy Fuels 2020, 4, 1730–1746. [Google Scholar] [CrossRef]
- Palm, R.; Kurig, H.; Tõnurist, K.; Jänes, A.; Lust, E. Electrical double layer capacitors based on 1-ethyl-3-methylimidazolium tetrafluoroborate with small addition of acetonitrile. Electrochim. Acta 2012, 85, 139–144. [Google Scholar] [CrossRef]
- Awale, D.V.; Bhise, S.C.; Patil, S.K.; Vadiyar, M.M.; Jadhav, P.R.; Navathe, G.J.; Kim, J.H.; Patil, P.S.; Kolekar, S.S. Nanopetals assembled copper oxide electrode for supercapacitor using novel 1-(1′-methyl-2′-oxo-propyl)-2,3-dimethylimidazolium chloride ionic liquid as an electrolyte. Ceram. Int. 2016, 42, 2699–2705. [Google Scholar] [CrossRef]
- Kim, E.; Han, J.; Ryu, S.; Choi, Y.; Yoo, J. Ionic Liquid Electrolytes for Electrochemical Energy Storage Devices. Materials 2021, 14, 400. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Ail, U.; Nadia Ajjan, F.; Phopase, J.; Ullah Khan, Z.; Kim, N.; Nilsson, J.; Inganäs, O.; Berggren, M.; Crispin, X. Water-in-Polymer Salt Electrolyte for Slow Self-Discharge in Organic Batteries. Adv. Energy Sustain. Res. 2021, 3, 2100165. [Google Scholar] [CrossRef]
- Lewandowski, A.; Galiński, M. Carbon–ionic liquid double-layer capacitors. J. Phys. Chem. Solids 2004, 65, 281–286. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.S. Ionic Liquid-Based Electrolytes for Energy Storage Devices: A Brief Review on Their Limits and Applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer. Science 2006, 313, 1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.E.; Jin, Z.; Wu, J. Oscillation of capacitance inside nanopores. Nano Lett. 2011, 11, 5373–5377. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, H.; Sheridan, E.; Walmsley, J.C.; Ren, D.; Chen, D. Geometrically confined favourable ion packing for high gravimetric capacitance in carbon–ionic liquid supercapacitors. Energy Environ. Sci. 2016, 9, 232–239. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Relation between the Ion Size and Pore Size for an Electric Double-Layer Capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef]
- Tang, X.; Xiao, D.; Xu, Z.; Liu, Q.; Ding, B.; Dou, H.; Zhang, X. A novel ionic liquid-based electrolyte assisting the high performance of low-temperature supercapacitors. J. Mater. Chem. A 2022, 10, 18374–18382. [Google Scholar] [CrossRef]
- Wang, X.; Mehandzhiyski, A.Y.; Arstad, B.; van Aken, K.L.; Mathis, T.S.; Gallegos, A.; Tian, Z.; Ren, D.; Sheridan, E.; Grimes, B.A.; et al. Selective Charging Behavior in an Ionic Mixture Electrolyte-Supercapacitor System for Higher Energy and Power. J. Am. Chem. Soc. 2017, 139, 18681–18687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yambou, E.P.; Gorska, B.; Beguin, F. Electrical Double-Layer Capacitors Based on a Ternary Ionic Liquid Electrolyte Operating at Low Temperature with Realistic Gravimetric and Volumetric Energy Outputs. ChemSusChem 2021, 14, 1196–1208. [Google Scholar] [CrossRef]
- Van Aken, K.L.; Beidaghi, M.; Gogotsi, Y. Formulation of ionic-liquid electrolyte to expand the voltage window of supercapacitors. Angew. Chem. Int. Ed. 2015, 54, 4806–4809. [Google Scholar] [CrossRef]
- Lian, C.; Liu, K.; van Aken, K.L.; Gogotsi, Y.; Wesolowski, D.J.; Liu, H.L.; Jiang, D.E.; Wu, J.Z. Enhancing the Capacitive Performance of Electric Double-Layer Capacitors with Ionic Liquid Mixtures. ACS Energy Lett. 2016, 1, 21–26. [Google Scholar] [CrossRef]
- Osti, N.C.; Gallegos, A.; Dyatkin, B.; Wu, J.; Gogotsi, Y.; Mamontov, E. Mixed Ionic Liquid Improves Electrolyte Dynamics in Supercapacitors. J. Phys. Chem. C 2018, 122, 10476–10481. [Google Scholar] [CrossRef]
- Vranes, M.; Dozic, S.; Djeric, V.; Gadzuric, S. Physicochemical Characterization of 1-Butyl-3-methylimidazolium and 1-Butyl-1-methylpyrrolidinium Bis(trifluoromethylsulfonyl)imide. J. Chem. Eng. Data 2012, 57, 1072–1077. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Patra, J.; Hsieh, C.T.; Li, J.; Dong, Q.F.; Chang, J.K. Supercapacitive Properties of Micropore- and Mesopore-Rich Activated Carbon in Ionic-Liquid Electrolytes with Various Constituent Ions. ChemSusChem 2019, 12, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Jackel, N.; Kruner, B.; van Aken, K.L.; Alhabeb, M.; Anasori, B.; Kaasik, F.; Gogotsi, Y.; Presser, V. Electrochemical in Situ Tracking of Volumetric Changes in Two-Dimensional Metal Carbides (MXenes) in Ionic Liquids. ACS Appl. Mater. Interfaces 2016, 8, 32089–32093. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ramirez, N.; Martins, V.L.; Ando, R.A.; Camilo, F.F.; Urahata, S.M.; Ribeiro, M.C.; Torresi, R.M. Physicochemical properties of three ionic liquids containing a tetracyanoborate anion and their lithium salt mixtures. J. Phys. Chem. B 2014, 118, 8772–8781. [Google Scholar] [CrossRef]
- Mezger, M.; Schröder, H.; Reichert, H.; Schramm, S.; Okasinski, J.S.; Schöder, S.; Honkimäki, V.; Deutsch, M.; Ocko, B.M.; Ralston, J.; et al. Molecular Layering of Fluorinated Ionic Liquids at a Charged Sapphire (0001) Surface. Science 2008, 322, 424–428. [Google Scholar] [CrossRef]
- Ye, C.; Shreeve, J.N.M. Rapid and Accurate Estimation of Densities of Room-Temperature Ionic Liquids and Salts. J. Phys. Chem. A. 2007, 111, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- Weingarth, D.; Noh, H.; Foelske-Schmitz, A.; Wokaun, A.; Kötz, R. A reliable determination method of stability limits for electrochemical double layer capacitors. Electrochim. Acta 2013, 103, 119–124. [Google Scholar] [CrossRef]
- Chen, Y.; Zhuo, K.; Chen, J.; Bai, G. Volumetric and viscosity properties of dicationic ionic liquids in (glucose + water) solutions at T = 298.15 K. J. Chem. Thermodyn. 2015, 86, 13–19. [Google Scholar] [CrossRef]
- Yambou, E.P.; Gorska, B.; Béguin, F. Binary mixtures of ionic liquids based on EMIm cation and fluorinated anions: Physico-chemical characterization in view of their application as low-temperature electrolytes. J. Mol. Liq. 2020, 298, 111959. [Google Scholar] [CrossRef]
- Fan, X.-H.; Chen, Y.-P.; Su, C.-S. Densities and viscosities of binary liquid mixtures of 1-ethyl-3-methylimidazolium tetrafluoroborate with acetone, methyl ethyl ketone, and N -methyl-2-pyrrolidone. J. Taiwan Inst. Chem E. 2016, 61, 117–123. [Google Scholar] [CrossRef]
- Hiraga, Y.; Kato, A.; Sato, Y.; Smith, R.L. Densities at Pressures up to 200 MPa and Atmospheric Pressure Viscosities of Ionic Liquids 1-Ethyl-3-methylimidazolium Methylphosphate, 1-Ethyl-3-methylimidazolium Diethylphosphate, 1-Butyl-3-methylimidazolium Acetate, and 1-Butyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide. J. Chem. Eng. Data 2015, 60, 876–885. [Google Scholar] [CrossRef]
- Shamsipur, M.; Beigi, A.A.M.; Teymouri, M.; Pourmortazavi, S.M.; Irandoust, M. Physical and electrochemical properties of ionic liquids 1-ethyl-3-methylimidazolium tetrafluoroborate, 1-butyl-3-methylimidazolium trifluoromethanesulfonate and 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide. J. Mol. Liq. 2010, 157, 43–50. [Google Scholar] [CrossRef]
- Vraneš, M.; Papović, S.; Tot, A.; Zec, N.; Gadžurić, S. Density, excess properties, electrical conductivity and viscosity of 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide+γ-butyrolactone binary mixtures. J. Chem. Thermodyn. 2014, 76, 161–171. [Google Scholar] [CrossRef]
- Widegren, J.A.; Saurer, E.M.; Marsh, K.N.; Magee, J.W. Electrolytic conductivity of four imidazolium-based room-temperature ionic liquids and the effect of a water impurity. J. Chem. Thermodyn. 2005, 37, 569–575. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Chen, Y.; Du, Q.; Zhuo, K.; Yang, L.; Sun, D.; Bai, G. Ionic Liquid Mixture Electrolyte Matching Porous Carbon Electrodes for Supercapacitors. Materials 2022, 15, 7400. https://doi.org/10.3390/ma15207400
Zhao Y, Chen Y, Du Q, Zhuo K, Yang L, Sun D, Bai G. Ionic Liquid Mixture Electrolyte Matching Porous Carbon Electrodes for Supercapacitors. Materials. 2022; 15(20):7400. https://doi.org/10.3390/ma15207400
Chicago/Turabian StyleZhao, Yuhua, Yujuan Chen, Quanzhou Du, Kelei Zhuo, Lifang Yang, Dong Sun, and Guangyue Bai. 2022. "Ionic Liquid Mixture Electrolyte Matching Porous Carbon Electrodes for Supercapacitors" Materials 15, no. 20: 7400. https://doi.org/10.3390/ma15207400
APA StyleZhao, Y., Chen, Y., Du, Q., Zhuo, K., Yang, L., Sun, D., & Bai, G. (2022). Ionic Liquid Mixture Electrolyte Matching Porous Carbon Electrodes for Supercapacitors. Materials, 15(20), 7400. https://doi.org/10.3390/ma15207400