Porous Carbon–Carbon Composite Materials Obtained by Alkaline Dehydrochlorination of Polyvinyl Chloride
Abstract
:1. Introduction
2. Materials and Methods
3. Results
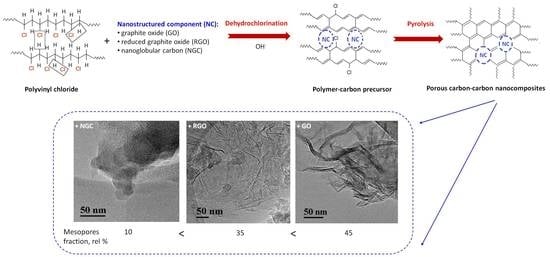
3.1. Synthesis of Carbon–Carbon Nanocomposites
3.2. A Structural Study of Carbon–Carbon Nanocomposites
3.3. Investigation of the Textural Characteristics of Carbon–Carbon Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.; Buekens, A.; Jiang, X.; Li, X. Dioxins and polyvinylchloride in combustion and fires. Waste Manag. Res. 2015, 33, 630–643. [Google Scholar] [CrossRef]
- Lipik, V.T.; Martsul’, V.N.; Abadie, M.J.M. Dehydrochlorination of PVC Compositions During Thermal Degradation. Eurasian Chem.-Technol. J. 2002, 4, 25–29. [Google Scholar] [CrossRef]
- Thornton, J. Environmental Impacts of Polyvinyl Chloride Building Materials; © Healthy Building Network: Washington, DC, USA, 2002. [Google Scholar]
- Miao, F.; Liu, Y.; Gao, M.; Yu, X.; Xiao, P.; Wang, M.; Wang, S.; Wang, X. Degradation of polyvinyl chloride microplastics via an electro-Fenton-like system with a TiO2/graphite cathode. J. Hazard. Mater. 2020, 399, 123023. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, T.; Yasuhara, A.; Katami, T. Reviews of Environmental Contamination and Toxicology; Springer: Cham, Switzerland, 2007; Volume 190, pp. 1–41. [Google Scholar]
- Sadat-Shojai, M.; Bakhshandeh, G.-R. Recycling of PVC wastes. Polym. Degrad. Stab. 2011, 96, 404–415. [Google Scholar] [CrossRef]
- Buekens, A.; Cen, K. Waste incineration, PVC, and dioxins. J. Mater. Cycles Waste Manag. 2011, 13, 190–197. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.; Majka, T. Thermal Degradation of Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Al-Enizi, A.M.; Ahmed, J.; Ubaidullah, M.; Shaikh, S.F.; Ahamad, T.; Naushad, M.; Zheng, G. Utilization of waste polyethylene terephthalate bottles to develop metal-organic frameworks for energy applications: A clean and feasible approach. J. Clean. Prod. 2020, 248, 119251. [Google Scholar] [CrossRef]
- Keane, M.A. Review. Catalytic conversion of waste plastics: Focus on waste PVC. J. Chem. Technol. Biotechnol. 2007, 82, 787–795. [Google Scholar] [CrossRef]
- Rathoure, A.K. Dioxins: Source, origin and toxicity assessment. Biodivers. Int. J. 2018, 2, 310–314. [Google Scholar] [CrossRef] [Green Version]
- Bowley, H.J.; Gerrard, D.L.; Maddams, W.F. Formation of conjugated polyenes by chemical and thermal degradation of vinyl chloride copolymers and other vinyl polymers. Die Makromol. Chem. 1985, 186, 715–723. [Google Scholar] [CrossRef]
- Li, T.; Zhao, P.; Lei, M.; Li, Z. Understanding Hydrothermal Dechlorination of PVC by Focusing on the Operating Conditions and Hydrochar Characteristics. Appl. Sci. 2017, 7, 256. [Google Scholar] [CrossRef]
- Ma, D.; Liang, L.; Hu, E.; Chen, H.; Wang, D.; He, C.; Feng, Q. Dechlorination of polyvinyl chloride by hydrothermal treatment with cupric ion. Process Saf. Environ. Prot. 2020, 146, 108–117. [Google Scholar] [CrossRef]
- Zakharyan, E.M.; Petrukhina, N.N.; Dzhabarov, E.G.; Maksimov, A.L. Pathways of Chemical Recycling of Polyvinyl Chloride. Part 2. Russ. J. Appl. Chem. 2020, 93, 1445–1490. [Google Scholar] [CrossRef]
- Okada, T.; Sutoh, S.; Sejima, K.; Tomohara, H.; Mishima, S. A useful method for thorough dehydrochlorination of Poly(vinylidene chloride-co-vinyl chloride) using Zinc(II) oxide. Polym. Degrad. Stab. 2020, 171, 109040. [Google Scholar] [CrossRef]
- Nishibata, H.; Uddin, M.A.; Kato, Y. Simultaneous degradation and dechlorination of poly (vinyl chloride) by a combination of superheated steam and CaO catalyst/adsorbent. Polym. Degrad. Stab. 2020, 179, 109225. [Google Scholar] [CrossRef]
- Chang, Y.; Dang, Q.; Samo, I.; Li, Y.; Li, X.; Zhang, G.; Chang, Z. Electrochemical heavy metal removal from water using PVC waste-derived N, S co-doped carbon. R. Soc. Chem. 2020, 10, 4064–4070. [Google Scholar] [CrossRef] [Green Version]
- Kryazhev, Y.G.; Solodovnichenko, V.S.; Antonicheva, N.V.; Drozdov, V.A. Evolution of the structures and sorption properties of dehydrochlorinated chloropolymers during their thermal conversions. Prot. Met. Phys. Chem. Surf. 2009, 45, 398–402. [Google Scholar] [CrossRef]
- Solodovnichenko, V.S.; Kryazhev, Y.G.; Arbuzov, A.B.; Talzi, V.P.; Antonicheva, N.V.; Drozdov, V.A.; Zapevalova, E.S.; Likholobov, V.A. Polyvinyl chloride as a precursor for low-temperature synthesis of carbon materials. Russ. Chem. Bull. 2016, 65, 2712–2717. [Google Scholar] [CrossRef]
- Kryazhev, Y.G.; Solodovnichenko, V.S.; Anikeeva, I.V.; Ismagilov, Z.R.; Pod’yacheva, O.Y.; Kvon, R.I.; Drozdov, V.A.; Likholobov, V.A. Synthesis and characterization of nanostructured hybrid nitrogen- and metal-containing carbon materials. Solid Fuel Chem. 2015, 49, 1–6. [Google Scholar] [CrossRef]
- Anikeeva, I.V.; Kryazhev, Y.G.; Solodovnichenko, V.S.; Drozdov, V.A. Preparation of composites with globular carbon nanoparticles distributed in an amorphous carbon matrix. Solid Fuel Chem. 2012, 46, 271–274. [Google Scholar] [CrossRef]
- Kryazhev, Y.G.; Volfkovich, Y.M.; Mel’nikov, V.P.; Rychagov, A.Y.; Trenikhin, M.V.; Solodovnichenko, V.S.; Zapevalova, E.S.; Likholobov, V.A. Synthesis and study of electrochemical properties of nanocomposites with graphene-like particles integrated into a high-porosity carbon matrix. Prot. Met. Phys. Chem. Surf. 2017, 53, 422–425. [Google Scholar] [CrossRef]
- Marsh, H.; Rodríguez-Reinoso, F. Activated Carbon; Copyright © Elsevier Ltd.: Amsterdam, The Netherlands, 2006. [Google Scholar] [CrossRef]
- Högberg, H.; Chung-Chuan Lai, C.-C.; Broitman, E.; Ivanov, I.G.; Goyenola, C.; Näslund, L.-A.; Schmidt, S.; Hultman, L.; Rosen, J.; Gueorguiev, G.K. Reactive sputtering of CSx thin solid films using CS2 as precursor. Vacuum 2020, 182, 109775. [Google Scholar] [CrossRef]
- Bakoglidis, K.D.; Palisaitis, J.; dos Santos, R.B.; Rivelino, R.; Persson, P.O.A.; Gueorguiev, G.K.; Hultman, L. Self-Healing in Carbon Nitride Evidenced As Material Inflation and Superlubric Behavior. ACS Appl. Mater. Interfaces 2018, 10, 16238–16243. [Google Scholar] [CrossRef] [Green Version]
- Bocharov, G.S.; Eletskii, A.V. Percolation Conduction of Carbon Nanocomposites. Int. J. Mol. Sci. 2020, 21, 7634. [Google Scholar] [CrossRef] [PubMed]
- El-Shamya, A.G.; Zayied, H.S.S. New polyvinyl alcohol/carbon quantum dots (PVA/CQDs) nanocomposite films: Structural, optical and catalysis properties. Synth. Met. 2020, 259, 116218. [Google Scholar] [CrossRef]
- Puech, P.; Kandara, M.; Paredes, G.; Moulin, L.; Weiss-Hortala, E.; Kundu, A.; Ratel-Ramond, N.; Plewa, J.-M.; Pellenq, R.; Monthioux, M. Analyzing the Raman Spectra of Graphenic Carbon Materials from Kerogens to Nanotubes: What Type of Information Can Be Extracted from Defect Bands? C 2009, 5, 69. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S. Preparation of Graphitic Acid. U.S. Patent No. 2,798,878, 7 September 1957. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Abed, N.A.; Jameel, W.W.; Hassan, N.S. Studying the Electrical Conductivity of Different Carbon Fillers Reinforced Polyvinyl Chloride Composite Materials. Nahrain Univ. Coll. Eng. J. (NUCEJ) 2014, 16, 260–268. [Google Scholar]
- Chung, K.T.; Sabo, A.; Pica, A.P. Electrical permittivity and conductivity of carbon black-polyvinyl chloride composites. J. Appl. Phys. 1982, 53, 6867. [Google Scholar] [CrossRef]
- Hui, D.; Chipara, M.; Lau, Κ.T.; Sankar, J.; Chipara, M.D.; Notingher, P. and Panaitescu, D. Investigations on Polyvinyl—Carbon Black Blends. Sci. Eng. Compos. Mater. 2004, 11, 19–26. [Google Scholar] [CrossRef]
- Ruslan, M.S.; Chew, S.P.; Sharif, M.; Azid, A.A.; Yusof, A. EMI Shielding Effectiveness of Polyvinyl Chloride and Carbon Fiber Composites in Building Construction. Adv. Mater. Res. 2014, 895, 452–459. [Google Scholar] [CrossRef]
- Nasouri, K.; Shoushtari, A.M. Designing, modeling and manufacturing of lightweight carbon nanotubes/polymer composite nanofibers for electromagnetic interference shielding application. Compos. Sci. Technol. 2017, 145, 46–54. [Google Scholar] [CrossRef]
- George, G.; Simon, S.M.; Prakashan, V.P.; Sajna, M.S.; Faisal, M.; Chandran, A.; Wilson, R.; Biju, P.R.; Joseph, C.; Unnikrishnan, N.V. Morphological, dielectric, tunable electromagnetic interference shielding and thermal characteristics of multiwalled carbon nanotube incorporated polymer nanocomposites: A facile, environmentally benign and cost effective approach realized via polymer latex/waterborne polymer as matrix. Polym. Compos. 2018, 39, E1169–E1183. [Google Scholar] [CrossRef]
- Wang, H.; Xie, G.; Fang, M.; Ying, Z.; Tong, Y.; Zeng, Y. Electrical and mechanical properties of antistatic PVC films containing multi-layer grapheme. Compos. Part B Eng. 2014, 79, 444–450. [Google Scholar] [CrossRef]
- Ahmad, N.; Kausar, A.; Muhammad, B. Perspectives on Polyvinyl Chloride and Carbon Nanofiller Composite: A Review. Polym.-Plast. Technol. Eng. 2016, 55, 1076–1098. [Google Scholar] [CrossRef]
- Naim, A.F.A.; AlFannakh, H.; Arafat, S.; Ibrahim, S.S. Characterization of PVC/MWCNTs Nanocomposite: Solvent Blend. Sci. Eng. Compos. Mater. 2020, 27, 55–64. [Google Scholar] [CrossRef]
- Yazdani, H.; Smith, B.E.; Hatami, K. Multi-walled carbon nanotube-filled polyvinyl chloride composites: Influence of processing method on dispersion quality, electrical conductivity and mechanical properties. Compos. Part A 2015, 82, 65–77. [Google Scholar] [CrossRef]
- Trommer, K.; Petzold, C.; Morgenstern, B. Processing and Properties of Carbon Nanotube PVC Composites. J. Appl. Chem. 2014, 2014, 307274. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, K.; Khatake, S.M.; Joshi, G.M. Surface properties of graphene oxide reinforced polyvinyl chloride nanocomposites. J. Polym. Res. 2013, 20, 286. [Google Scholar] [CrossRef]
- Arani, F.; Mirhabibi, A. Effect of nano carbon additives on the microstructure of polyvinyl chloride heated up to 2000 °C. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 34–42. [Google Scholar] [CrossRef]
- Pramanik, C.; Nepal, D.; Nathanson, M.; Gissinger, J.R.; Garley, A.; Berry, R.J.; Davijani, A.; Kumar, S.; Heinz, H. Molecular engineering of interphases in polymer/carbon nanotube composites to reach the limits of mechanical performance. Compos. Sci. Technol. 2018, 166, 86–94. [Google Scholar] [CrossRef]
- Sapalidis, A.; Sideratou, Z.; Panagiotaki, K.N.; Sakellis, E.; Kouvelos, E.P.; Papageorgiou, S.; Katsaros, F. Fabrication of Antibacterial Poly(Vinyl Alcohol) Nanocomposite Films Containing Dendritic Polymer Functionalized Multi-Walled Carbon Nanotubes. Front. Mater. 2018, 5, 11. [Google Scholar] [CrossRef]
- Sabet, S.M.; Mahfuz, H.; Terentis, A.C.; Nezakat, M. Effects of POSS functionalization of carbon nanotubes on microstructure and thermomechanical behavior of carbon nanotube/polymer nanocomposites. J. Mater. Sci. 2018, 53, 8963–8977. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, H.; Xu, R. Research on the dispersion of carbon nanotubes by ultrasonic oscillation, surfactant and centrifugation respectively and fiscal policies for its industrial development. Ultrason. Sonochem. 2018, 48, 30–38. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.; Fu, X.; Wang, Y.; Zhong, W.H. A bio-surfactant for defect control: Multifunctional gelatin coated MWCNTs for conductive epoxy nanocomposites. Compos. Sci. Technol. 2018, 159, 216–224. [Google Scholar] [CrossRef]
- Yu, T.; Herrera, J.E. The Mechanism of Surfactant Assisted Dispersion of Single-Walled Carbon Nanotubes in Polyvinylpyrrolidone Solutions. Colloid Surf. Sci. 2017, 2, 96–106. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET equation applicable to microporous adsorbents? Stud. Surf. Sci. Catal. 2007, 160, 49–56. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodrigues-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Barrett, E.P.; Joiner, L.G.; Halenda, P.H. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373. [Google Scholar] [CrossRef]
- Tarazona, P.; Marconi, U.M.B.; Evans, R. Phase equilibria of fluid interfaces and confined fluids. Mol. Phys. 1987, 60, 573–595. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A. Physical adsorption characterization of nanoporous materials: Progress and challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Zhu, W.; Miser, D.E.; Chan, W.G.; Hajaligol, M.R. HRTEM investigation of some commercially available furnace carbon blacks. Carbon 2004, 42, 1841–1845. [Google Scholar] [CrossRef]
- Yehliu, K.; Vander Wal, R.L.; Boehman, A.L. Development of an HRTEM image analysis method to quantify carbon nanostructure. Combust. Flame 2011, 158, 1837–1851. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Jiang, S.; Hou, H. Carbonization: A feasible route for reutilization of plastic wastes. Sci. Total Environ. 2020, 710, 136250. [Google Scholar] [CrossRef] [PubMed]
- Wypych, G. PVC Degradation and Stabilization. II. In PVC Morphology; Part 3; ChemTec Publishing: Scarborough, ON, Canada, 2015; pp. 47–78. [Google Scholar] [CrossRef]
- Gudkov, M.V.; Bazhenov, S.L.; Bekhli, L.S.; Mel’nikov, V.P. Explosive Reduction of Graphite Oxide. Rus. J. Phys. Chem. B 2018, 12, 860–868. [Google Scholar] [CrossRef]









| Modifying Additive (NC Content, wt.% 1) | SBET, m2 g−1 | Vads, cm3 g−1 | Vmicro, cm3 g−1 | Fraction of Meso (Macro) Pores, rel. % | |
|---|---|---|---|---|---|
| t-Method | NLDFT | ||||
| - | 1102 | 0.47 | 0.40 | 0.42 | 15 |
| NGC (6) | 791 | 0.35 | 0.31 | 0.30 | 11 |
| GO (5) | 1115 | 0.70 | 0.38 | 0.41 | 46 |
| RGO (7) | 979 | 0.55 | 0.35 | 0.37 | 36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryazhev, Y.G.; Anikeeva, I.V.; Trenikhin, M.V.; Gulyaeva, T.I.; Melnikov, V.P.; Likholobov, V.A.; Belskaya, O.B. Porous Carbon–Carbon Composite Materials Obtained by Alkaline Dehydrochlorination of Polyvinyl Chloride. Materials 2022, 15, 7636. https://doi.org/10.3390/ma15217636
Kryazhev YG, Anikeeva IV, Trenikhin MV, Gulyaeva TI, Melnikov VP, Likholobov VA, Belskaya OB. Porous Carbon–Carbon Composite Materials Obtained by Alkaline Dehydrochlorination of Polyvinyl Chloride. Materials. 2022; 15(21):7636. https://doi.org/10.3390/ma15217636
Chicago/Turabian StyleKryazhev, Yury G., Irina V. Anikeeva, Mikhail V. Trenikhin, Tatiana I. Gulyaeva, Valeriy P. Melnikov, Vladimir A. Likholobov, and Olga B. Belskaya. 2022. "Porous Carbon–Carbon Composite Materials Obtained by Alkaline Dehydrochlorination of Polyvinyl Chloride" Materials 15, no. 21: 7636. https://doi.org/10.3390/ma15217636
APA StyleKryazhev, Y. G., Anikeeva, I. V., Trenikhin, M. V., Gulyaeva, T. I., Melnikov, V. P., Likholobov, V. A., & Belskaya, O. B. (2022). Porous Carbon–Carbon Composite Materials Obtained by Alkaline Dehydrochlorination of Polyvinyl Chloride. Materials, 15(21), 7636. https://doi.org/10.3390/ma15217636