Improving the Performance of Solution−Processed Quantum Dot Light−Emitting Diodes via a HfOx Interfacial Layer
Abstract
:1. Introduction
2. Experimental Section
2.1. Solutions Preparation
2.2. Device Fabrication
2.3. Characterization
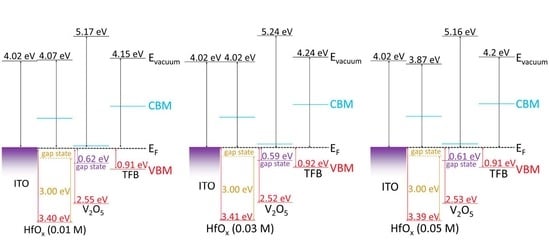
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, H.; Lee, C.; Lee, W.; Kim, J.; Chae, H. Stability of Quantum Dots, Quantum Dot Films, and Quantum Dot Light−Emitting Diodes for Display Applications. Adv. Mater. 2019, 31, 1804294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, C.; He, T.; Jiang, Y.; Wei, J.; Huang, Y.; Yuan, M. High−Performance Quasi−2D Perovskite Light−Emitting Diodes: From Materials to Devices. Light Sci. Appl. 2021, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Anaya, M.; Abfalterer, A.; Stranks, S.D. Halide Perovskite Light−Emitting Diode Technologies. Adv. Opt. Mater. 2021, 9, 2002128. [Google Scholar] [CrossRef]
- Sudheendran Swayamprabha, S.; Dubey, D.K.; Shahnawaz; Yadav, R.A.K.; Nagar, M.R.; Sharma, A.; Tung, F.; Jou, J. Approaches for Long Lifetime Organic Light Emitting Diodes. Adv. Sci. 2020, 8, 2002254. [Google Scholar] [CrossRef]
- Cheng, Y.; Wan, H.; Liang, T.; Liu, C.; Wu, M.; Hong, H.; Liu, K.; Shen, H. Continuously Graded Quantum Dots: Synthesis, Applications in Quantum Dot Light−Emitting Diodes, and Perspectives. J. Phys. Chem. Lett. 2021, 12, 5967–5978. [Google Scholar] [CrossRef]
- Won, Y.-H.; Cho, O.; Kim, T.; Chung, D.-Y.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly Efficient and Stable InP/ZnSe/ZnS Quantum Dot Light−Emitting Diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef]
- Park, M.; Roh, J.; Lim, J.; Lee, H.; Lee, D. Double Metal Oxide Electron Transport Layers for Colloidal Quantum Dot Light−Emitting Diodes. Nanomaterials 2020, 10, 726. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Moon, H.; Lee, W.; Hwangbo, H.; Yong, S.H.; Chung, H.K.; Chae, H. Charge Balance Control of Quantum Dot Light Emitting Diodes with Atomic Layer Deposited Aluminum Oxide Interlayers. RSC Adv. 2019, 9, 11634–11640. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Sun, W.; Liu, P.; Wang, Z.; Zhang, J.; Wei, J.; Li, Y.; Hayat, T.; Alsaedi, A.; Tan, Z. Achieving Balanced Charge Injection of Blue Quantum Dot Light−Emitting Diodes through Transport Layer Doping Strategies. J. Phys. Chem. Lett. 2019, 10, 960–965. [Google Scholar] [CrossRef]
- Jin, X.; Chang, C.; Zhao, W.; Huang, S.; Gu, X.; Zhang, Q.; Li, F.; Zhang, Y.; Li, Q. Balancing the Electron and Hole Transfer for Efficient Quantum Dot Light−Emitting Diodes by Employing a Versatile Organic Electron−Blocking Layer. ACS Appl. Mater. Interfaces 2018, 10, 15803–15811. [Google Scholar] [CrossRef]
- Zhang, N.; Qu, X.; Lyu, Q.; Wang, K.; Sun, X.W. Highly Efficient Transparent Quantum−Dot Light−Emitting Diodes Based on Inorganic Double Electron−Transport Layers. Photonics Res. 2021, 9, 1979–1983. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, W.; Wu, Y.; He, Z.; Zhang, S.; Chen, S. A Low−Temperature−Annealed and UV−Ozone−Enhanced Combustion Derived Nickel Oxide Hole Injection Layer for Flexible Quantum Dot Light−Emitting Diodes. Nanoscale 2019, 11, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Chakraborty, P.; Majumder, T.; Mondal, S.P. Acid−Treated PEDOT:PSS Polymer and TiO2 Nanorod Schottky Junction Ultraviolet Photodetectors with Ultrahigh External Quantum Efficiency, Detectivity, and Responsivity. ACS Appl. Mater. Interfaces 2018, 10, 41618–41626. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, Y.R.; Lee, G.; Jin, S.W.; Lee, Y.H.; Hong, S.Y.; Park, H.; Kim, J.W.; Lee, S.-S.; Ha, J.S. Highly Conductive, Stretchable, and Transparent PEDOT:PSS Electrodes Fabricated with Triblock Copolymer Additives and Acid Treatment. ACS Appl. Mater. Interfaces 2018, 10, 28027–28035. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Kim, M.; Ma, J.H.; Jeong, J.H.; Hwang, H.W.; Kim, J.W.; Kang, S.J. Solution−processable Li−doped transition metal oxide hole−injection layer for highly efficient quantum−dot light−emitting diodes. J. Mater. Chem. C 2022, 10, 5590–5597. [Google Scholar] [CrossRef]
- Heo, S.B.; Yu, J.H.; Shin, J.S.; Kim, T.Y.; Kim, B.S.; Jeon, W.; Kang, S.J. Effect of Inorganic Interfacial Modification Layer on the Performance of Quantum−Dots Light−Emitting Diodes. Jpn. J. Appl. Phys. 2020, 59, 124002. [Google Scholar] [CrossRef]
- Shin, J.S.; Kim, T.Y.; Heo, S.B.; Hong, J.-A.; Park, Y.; Kang, S.J. Improving the Performance of Quantum−Dot Light−Emitting Diodes via an Organic–Inorganic Hybrid Hole Injection Layer. RSC Adv. 2021, 11, 4168–4172. [Google Scholar] [CrossRef]
- Kim, T.Y.; Park, S.; Kim, B.J.; Heo, S.B.; Yu, J.H.; Shin, J.S.; Hong, J.-A.; Kim, B.-S.; Kim, Y.D.; Park, Y.; et al. Dual−Functional Quantum−Dots Light Emitting Diodes Based on Solution Processable Vanadium Oxide Hole Injection Layer. Sci. Rep. 2021, 11, 1700. [Google Scholar] [CrossRef]
- Park, S.; Kim, B.J.; Kang, S.J.; Cho, N.-K. Photocurrent Characteristics of Zinc−Oxide Films Prepared by Using Sputtering and Spin−Coating Methods. J. Korean Phys. Soc. 2018, 73, 1351–1355. [Google Scholar] [CrossRef]
- Selvakumar, N.; Barshilia, H.C.; Rajam, K.S.; Biswas, A. Structure, Optical Properties and Thermal Stability of Pulsed Sputter Deposited High Temperature HfOx/Mo/HfO2 Solar Selective Absorbers. Sol. Energy Mater. Sol. Cells 2010, 94, 1412–1420. [Google Scholar] [CrossRef]
- Paul, A.D.; Biswas, S.; Das, P.; Edwards, H.J.; Dalal, A.; Maji, S.; Dhanak, V.R.; Mondal, A.; Mahapatra, R. Improved Resistive Switching Characteristics of Ag/Al:HfOx/ITO/PET ReRAM for Flexible Electronics Application. Semicond. Sci. Technol. 2021, 36, 065006. [Google Scholar] [CrossRef]
- Kim, M.G.; Shin, J.S.; Ma, J.H.; Jeong, J.H.; Han, D.H.; Kim, B.-S.; Jeon, W.; Park, Y.; Kang, S.J. An Al−Doped TiO2 Interfacial Layer for Effective Hole Injection Characteristics of Quantum−Dot Light−Emitting Diodes. J. Mater. Chem. C 2022, 10, 7294–7303. [Google Scholar] [CrossRef]
- Narayanan, R.; Dewan, A.; Chakraborty, D. Complimentary Effects of Annealing Temperature on Optimal Tuning of Functionalized Carbon–V2O5 Hybrid Nanobelts for Targeted Dual Applications in Electrochromic and Supercapacitor Devices. RSC Adv. 2018, 8, 8596–8606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kita, K.; Toriumi, A. Origin of Electric Dipoles Formed at High−k/SiO2 Interface. Appl. Phys. Lett. 2009, 94, 132902. [Google Scholar] [CrossRef]
- Hadacek, N.; Nosov, A.; Ranno, L.; Strobel, P.; Galéra, R.-M. Magnetic Properties of HfO2Thin Films. J. Phys. Condens. Matter 2007, 19, 486206. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, E.; Kurian, J.; Müller, M.M.; Schroeder, T.; Kleebe, H.-J.; Alff, L. Controlled Oxygen Vacancy Induced p−Type Conductivity in HfO2−x Thin Films. Appl. Phys. Lett. 2011, 99, 112902. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, N.; Vogel, T.; Zintler, A.; Petzold, S.; Arzumanov, A.; Piros, E.; Eilhardt, R.; Molina−Luna, L.; Alff, L. Defect−Stabilized Substoichiometric Polymorphs of Hafnium Oxide with Semiconducting Properties. ACS Appl. Mater. Interfaces 2021, 14, 1290–1303. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.H.; Kim, M.G.; Ma, J.H.; Park, M.H.; Ha, H.J.; Kang, S.J.; Maeng, M.-J.; Kim, Y.D.; Park, Y.; Kang, S.J. Improving the Performance of Solution−Processed Quantum Dot Light−Emitting Diodes via a HfOx Interfacial Layer. Materials 2022, 15, 8977. https://doi.org/10.3390/ma15248977
Jeong JH, Kim MG, Ma JH, Park MH, Ha HJ, Kang SJ, Maeng M-J, Kim YD, Park Y, Kang SJ. Improving the Performance of Solution−Processed Quantum Dot Light−Emitting Diodes via a HfOx Interfacial Layer. Materials. 2022; 15(24):8977. https://doi.org/10.3390/ma15248977
Chicago/Turabian StyleJeong, Jun Hyung, Min Gye Kim, Jin Hyun Ma, Min Ho Park, Hyoun Ji Ha, Seong Jae Kang, Min-Jae Maeng, Young Duck Kim, Yongsup Park, and Seong Jun Kang. 2022. "Improving the Performance of Solution−Processed Quantum Dot Light−Emitting Diodes via a HfOx Interfacial Layer" Materials 15, no. 24: 8977. https://doi.org/10.3390/ma15248977
APA StyleJeong, J. H., Kim, M. G., Ma, J. H., Park, M. H., Ha, H. J., Kang, S. J., Maeng, M. -J., Kim, Y. D., Park, Y., & Kang, S. J. (2022). Improving the Performance of Solution−Processed Quantum Dot Light−Emitting Diodes via a HfOx Interfacial Layer. Materials, 15(24), 8977. https://doi.org/10.3390/ma15248977