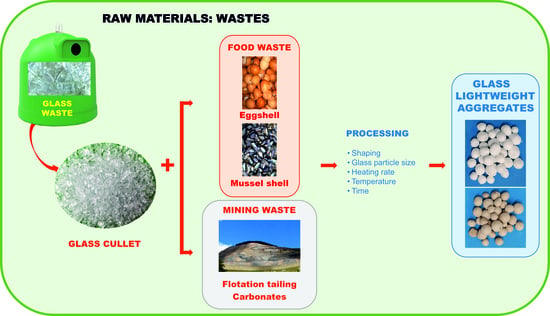
Glass Lightweight Aggregates from Glass Cullet and Mining and Food Industry Carbonate Waste
Abstract
:1. Introduction
2. Materials and Methods
- -
- Base glass particle size: <63 µm, 63–100 µm, 100–250 µm, 250–500 µm and <1 mm.
- -
- Percentage of foaming agent: 2.5, 5, 7.5, 10 and 15% by weight.
- -
- Type of shaping: The shaping of specimens by pressing and hand shaping was evaluated. For the preparation of pressed pellets, cylindrical specimens (2 cm diameter and approximately 1.5 cm in height) were formed from 2 g of a homogenized mixture of raw materials (glass cullet and foaming additive) slightly moistened (3% water). The shaping was performed by uniaxial pressing at 3 MPa for 30 s in a semiautomatic hydraulic press (Mignon–S model Nannetti S.r.l., Faenza, Italy). For hand shaping, 30 g of raw material mixtures was prepared and mixed with water (23% by weight). Spherical specimens (~1 cm in diameter and ~0.75 g in weight) were formed from each mixture. Prior to the foaming process, the specimens were dried at 105 °C for 24 h.
- -
- Heating rate: 10, 20, 30, 40 and 50 °C/min. In addition, the heating effect was evaluated by thermal shock, which consisted of introducing the samples into an oven preheated to the blowing temperature.
- -
- Firing temperature: 700, 800, 900 and 1000 °C.
- -
- Foaming time: 5, 10, 15, 20, 30 and 45 min.
3. Results and Discussion
3.1. Waste Characterization
3.2. Pressing–Formed Pellets
3.3. Hand–Formed Pellets
4. Conclusions
- -
- The expansion capacity of the different residues used as additives depends on the relative position of the onset decomposition temperature of the carbonates with respect to the glass transition temperature of the base glass.
- -
- All the studied blowing agents are effective in promoting the expansion of the specimens, with eggshell, mussel shell and flotation tailings being the most effective.
- -
- The heating rate does not produce a significant effect on the expansion values. Thermal shock heating is shown to be the most effective procedure for obtaining glass lightweight aggregates.
- -
- The mineralogical composition of the blowing agent and the particle size of the base glass are the two processing parameters that have the greatest influence on the degree of expansion.
- -
- All the foaming additives investigated in this study give rise to glass lightweight aggregates, with 800 °C being the most suitable processing temperature for the materials obtained to have the appropriate density and strength properties for their application as lightweight aggregates.
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanz-Pont, D.; Sanz-Arauz, D.; Bedoya-Frutos, C.; Flatt, R.J.; López-Andrés, S. Anhydrite/aerogel composites for thermal insulation. Mater. Struct. Constr. 2016, 49, 3647–3661. [Google Scholar] [CrossRef] [Green Version]
- UEPG. UEPG Annual Review 2019–2020. Eur. Aggregates Assoc. 2020, 1–31. [Google Scholar]
- Ellen MacArthur Foundation. Hacia una Economía Circular—Resumen Ejecutivo. 2014; 1–14. [Google Scholar]
- Glass Alliance Europe. Available online: https://www.glassallianceeurope.eu/en/homepage (accessed on 2 December 2021).
- Harder, J. Glass Recycling—Current Market Trends. 2018. Available online: https://www.recovery–worldwide.com/en/artikel/glass–recycling–current–market–trends_3248774.html (accessed on 15 December 2021).
- Eurostat Waste Generation by Packaging Material. Available online: https://ec.europa.eu/eurostat/statistics–explained/index.php?title=Packaging_waste_statistics#Waste_generation_by_packaging_material (accessed on 20 October 2021).
- FAO Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/poultry–production–products/production/en/ (accessed on 20 October 2021).
- Araneda, M. Huevos y Derivados. Composición y Propiedades. Available online: https://www.edualimentaria.com/huevos–composicion–y–propiedades (accessed on 20 October 2021).
- FAO Information and Analysis on World Fish Trade. Available online: https://www.fao.org/in–action/globefish/marketreports/resource–detail/es/c/522565/ (accessed on 30 November 2021).
- Lima, D.S.; Perez–Lopez, O.W. Oxidative coupling of methane to light olefins using waste eggshell as catalyst. Inorg. Chem. Commun. 2020, 116, 107928. [Google Scholar] [CrossRef]
- Bharti, R.; Guldhe, A.; Kumar, D.; Singh, B. Solar irradiation assisted synthesis of biodiesel from waste cooking oil using calcium oxide derived from chicken eggshell. Fuel 2020, 273, 117778. [Google Scholar] [CrossRef]
- Patil, U.P.; Patil, R.C.; Patil, S.S. Waste mussel shell as a highly efficient heterogeneous catalyst for the synthesis of polyfunctionalized 4H–pyrans in aqueous media. React. Kinet. Mech. Catal. 2020, 129, 679–691. [Google Scholar] [CrossRef]
- Tutus, A.; Killi, U.; Cicekler, M. Evaluation of eggshell wastes in office paper production. Biomass Convers. Biorefinery 2020, 1–10. [Google Scholar] [CrossRef]
- Abdullah, M.; Yoke, S.K.; Raofuddin, D.N.A.; Sukor, M.Z.; Roslan, A.; Ilyas, S.M.M.; Yasin, M.H. An evaluation of eggshell waste/waste paper mechanical properties as composite paper. Int. J. Eng. Technol. 2018, 7, 239–241. [Google Scholar] [CrossRef]
- Topal, M.; Arslan Topal, E.I. Optimization of tetracycline removal with chitosan obtained from mussel shells using RSM. J. Ind. Eng. Chem. 2020, 84, 315–321. [Google Scholar] [CrossRef]
- Bremner, C.; Cochrane, T.A.; McGuigan, P.; Bello–Mendoza, R. Removal of dissolved heavy metals from stormwater by filtration with granular recycled glass and mussel shell with and without microalgae biofilm. Environ. Technol. Innov. 2020, 18, 100662. [Google Scholar] [CrossRef]
- Martínez–García, C.; González–Fonteboa, B.; Carro–López, D.; Martínez–Abella, F. Design and properties of cement coating with mussel shell fine aggregate. Constr. Build. Mater. 2019, 215, 494–507. [Google Scholar] [CrossRef]
- Meski, S.; Tazibt, N.; Khireddine, H.; Ziani, S.; Biba, W.; Yala, S.; Sidane, D.; Boudjouan, F.; Moussaoui, N. Synthesis of hydroxyapatite from mussel shells for effective adsorption of aqueous Cd(II). Water Sci. Technol. 2019, 80, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Trakoolwannachai, V.; Kheolamai, P.; Ummartyotin, S. Characterization of hydroxyapatite from eggshell waste and polycaprolactone (PCL) composite for scaffold material. Compos. Part B Eng. 2019, 173, 106974. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Daniel, T.; Henry, J.; Sivakumar, G.; Mohanraj, K. Electrochemical performances of activated carbon prepared using eggshell waste. SN Appl. Sci. 2019, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rangel, E.M.; De Melo, C.C.N.; Carvalho, C.D.O.; Osorio, A.G.; Machado, F.M. Espumas vítreas produzidas a partir de resíduos sólidos. Rev. Mater. 2018, 23. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Ferreira, D.D.; Andreola, F.; Lancellotti, I.; Barbieri, L.; Ferreira, J.M.F. Environmental friendly management of CRT glass by foaming with waste egg shells, calcite or dolomite. Ceram. Int. 2014, 40, 13371–13379. [Google Scholar] [CrossRef]
- Zhang, Q.; He, F.; Shu, H.; Qiao, Y.; Mei, S.; Jin, M.; Xie, J. Preparation of high strength glass ceramic foams from waste cathode ray tube and germanium tailings. Constr. Build. Mater. 2016, 111, 105–110. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Petersen, R.R.; König, J.; Johra, H.; Yue, Y. Influence of foaming agents on solid thermal conductivity of foam glasses prepared from CRT panel glass. J. Non. Cryst. Solids 2017, 465, 59–64. [Google Scholar] [CrossRef]
- Petersen, R.R.; König, J.; Yue, Y. Evaluation of Foaming Behavior of Glass Melts by High–Temperature Microscopy. Int. J. Appl. Glas. Sci. 2016, 7, 524–531. [Google Scholar] [CrossRef]
- Ewais, E.M.M.; Attia, M.A.A.; El–Amir, A.A.M.; Elshenway, A.M.H.; Fend, T. Optimal conditions and significant factors for fabrication of soda lime glass foam from industrial waste using nano AlN. J. Alloys Compd. 2018, 747, 408–415. [Google Scholar] [CrossRef]
- Da Silva, R.C.; Kubaski, E.T.; Tenório–Neto, E.T.; Lima–Tenório, M.K.; Tebcherani, S.M. Foam glass using sodium hydroxide as foaming agent: Study on the reaction mechanism in soda–lime glass matrix. J. Non. Cryst. Solids 2019, 511, 177–182. [Google Scholar] [CrossRef]
- Arcaro, S.; De Maia, B.G.O.; Souza, M.T.; Cesconeto, F.R.; Granados, L.; De Oliveira, A.P.N. Thermal insulating foams produced from glass waste and banana leaves. Mater. Res. 2016, 19, 1064–1069. [Google Scholar] [CrossRef] [Green Version]
- Mariosi, F.R.; Camaratta, R.; Machado, F.M.; Rodrigues, L.F., Jr. Desenvolvimento de espumas vítreas a partir de garrafa e casca de ovo. Matéria (Rio Janeiro) 2019, 24. [Google Scholar] [CrossRef]
- Sooksaen, P.; Sudyod, N.; Thongtha, N.; Simsomboonphol, R. Fabrication of lightweight foam glasses for thermal insulation applications. Mater. Today Proc. 2019, 17, 1823–1830. [Google Scholar] [CrossRef]
- Tulyaganov, D.U.; Fernandes, H.R.; Agathopoulos, S.; Ferreira, J.M.F. Preparation and characterization of high compressive strength foams from sheet glass. J. Porous Mater. 2006, 13, 133–139. [Google Scholar] [CrossRef]
- Ercenk, E. The effect of clay on foaming and mechanical properties of glass foam insulating material. J. Therm. Anal. Calorim. 2017, 127, 137–146. [Google Scholar] [CrossRef]
- Bernardo, E.; Scarinci, G.; Bertuzzi, P.; Ercole, P.; Ramon, L. Recycling of waste glasses into partially crystallized glass foams. J. Porous Mater. 2010, 17, 359–365. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Iversen, N.; Yue, Y. Suppressing the effect of cullet composition on the formation and properties of foamed glass. Ceram. Int. 2018, 44, 11143–11150. [Google Scholar] [CrossRef]
- Akai, T.; Fukumi, K.; Yamashita, M. Formation of pale foam glass from colored glass cullet. J. Ceram. Soc. Japan 2020, 128, 153–157. [Google Scholar] [CrossRef]
- Ji, R.; Zheng, Y.; Zou, Z.; Chen, Z.; Wei, S.; Jin, X.; Zhang, M. Utilization of mineral wool waste and waste glass for synthesis of foam glass at low temperature. Constr. Build. Mater. 2019, 215, 623–632. [Google Scholar] [CrossRef]
- Qu, Y.-N.; Huo, W.-L.; Xi, X.-Q.; Gan, K.; Ma, N.; Hou, B.-Z.; Su, Z.-G.; Yang, J.-L. High porosity glass foams from waste glass and compound blowing agent. J. Porous Mater. 2016, 23, 1451–1458. [Google Scholar] [CrossRef]
- Petersen, R.R.; König, J.; Yue, Y. The viscosity window of the silicate glass foam production. J. Non. Cryst. Solids 2017, 456, 49–54. [Google Scholar] [CrossRef]
- Souza, M.T.; Maia, B.G.O.; Teixeira, L.B.; de Oliveira, K.G.; Teixeira, A.H.B.; Novaes de Oliveira, A.P. Glass foams produced from glass bottles and eggshell wastes. Process Saf. Environ. Prot. 2017, 111, 60–64. [Google Scholar] [CrossRef]
- Gong, Y.; Dongol, R.; Yatongchai, C.; Wren, A.W.; Sundaram, S.K.; Mellott, N.P. Recycling of waste amber glass and porcine bone into fast sintered and high strength glass foams. J. Clean. Prod. 2016, 112, 4534–4539. [Google Scholar] [CrossRef]
- Arriagada, C.; Navarrete, I.; Lopez, M. Understanding the effect of porosity on the mechanical and thermal performance of glass foam lightweight aggregates and the influence of production factors. Constr. Build. Mater. 2019, 228, 116746. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Petersen, R.R.; König, J.; Yue, Y. Effect of alkali phosphate content on foaming of CRT panel glass using Mn3O4 and carbon as foaming agents. J. Non. Cryst. Solids 2018, 482, 217–222. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, S.; Pan, X.; Zhou, S.; Zhao, M. Foaming mechanism and optimal process conditions of foamed glass based on thermal analysis. J. Porous Mater. 2020, 27, 621–626. [Google Scholar] [CrossRef]
- Dos Santos, P.A.M.; Priebbnow, A.V.; Arcaro, S.; da Silva, R.M.; Lopez, D.A.R.; Rodriguez, A.D.A.L. Sustainable Glass Foams Produced from Glass Bottles and Tobacco Residue. Mater. Res. 2018, 22, 11–12. [Google Scholar] [CrossRef]
- CEN. EN 658–2:2002 Advanced Technical Ceramics—Mechanical Properties of Ceramic Composites at Room Temperature—Part 2: Determination of Compression Properties; CEN—Comité Européen de Normalisation: Brussels, Belgium, 2002. [Google Scholar]
- Padilla, I.; Romero, M.; Robla, J.I.; López–Delgado, A. Waste and solar energy: An eco–friendly way for glass melting. ChemEngineering 2021, 5, 16. [Google Scholar] [CrossRef]
- Valverde, J.M.; Perejon, A.; Medina, S.; Perez-Maqueda, L.A. Thermal decomposition of dolomite under CO2: Insights from TGA and in situ XRD analysis. Phys. Chem. Chem. Phys. 2015, 17, 30162–30176. [Google Scholar] [CrossRef] [Green Version]
- Rat’Ko, A.I.; Ivanets, A.I.; Kulak, A.I.; Morozov, E.A.; Sakhar, I.O. Thermal decomposition of natural dolomite. Inorg. Mater. 2011, 47, 1372–1377. [Google Scholar] [CrossRef]
- Yoshioka, S.; Kitano, Y. Transformation of aragonite to calcite through heating. Geochem. J. 1985, 19, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Amoros, J.L.; Orts, M.J.; Garcia–Ten, J.; Gozalbo, A.; Sánchez, E. Effect of the green porous texture on porcelain tile properties. J. Eur. Ceram. Soc. 2007, 27, 2295–2301. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Yue, Y. Influence of the glass particle size on the foaming process and physical characteristics of foam glasses. J. Non. Cryst. Solids 2016, 447, 190–197. [Google Scholar] [CrossRef]









| Fixed Parameters | ||||||
|---|---|---|---|---|---|---|
| Variable Parameter | Shaping | Glass Particle Size | Foaming Agent (wt. %) | Heating Rate (°C/min) | Foaming Temperature (°C) | Foaming Time (min) |
| Foaming temp | Pressing | <1 mm | 5 | Thermal shock | Variable | 15 |
| Glass particle size | Pressing | Variable | 5 | Thermal shock | 700, 800, 900, 1000 | 15 |
| Heating rate | Pressing | <1 mm | 5 | Variable | 900 | 15 |
| Foaming time | Pressing | <1 mm | 5 | 10, 20, 30, 40, 50 | 900 | Variable |
| Foaming temp | Handly | <63 µm | 2.5, 5, 7.5, 10, 15 | Thermal shock | Variable | 15 |
| Foaming agent percentage | Handly | <63 µm | Variable | Thermal shock | 800, 900, 1000 | 15 |
| Foaming agent | Handly | <63 µm | 10 (*) | Thermal shock | 800, 850, 900 | Variable |
| Oxide | Glass Cullet (GC) | Carbonate F (CF) | Flotation Tailing (FT) | Carbonate PC8 (PC8) | Eggshell (ES) * | Mussel Shell (MS) * |
|---|---|---|---|---|---|---|
| SiO2 | 72.17 | 14.70 | 9.52 | 3.63 | 0.13 | 0.19 |
| Al2O3 | 1.84 | 3.41 | 1.18 | 0.83 | - | - |
| CaO | 15.04 | 17.44 | 32.27 | 11.85 | 97.24 | 97.68 |
| MgO | 0.30 | 61.93 | 54.96 | 61.40 | 0.40 | 0.10 |
| Na2O | 9.81 | 1.62 | 1.49 | 3.10 | - | 0.30 |
| K2O | 0.66 | 0.90 | 0.59 | 1.74 | 0.67 | 0.70 |
| SO3 | 0.17 | - | - | 10.08 | 0.98 | 0.45 |
| Fe2O3 | 0.00 | 7.11 | 5.48 | 5.28 | - | - |
| Cl | - | - | - | 1.07 | 0.27 | 0.28 |
| SrO | - | - | - | - | - | 0.20 |
| P2O5 | - | - | - | 0.61 | 0.21 | - |
| MnO | - | - | - | 0.15 | - | - |
| V2O5 | - | - | - | 0.14 | - | - |
| LOI | 2.02 | 50.67 | 49.59 | 12.89 | 47.00 | 46.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla, I.; López-Delgado, A.; Romero, M. Glass Lightweight Aggregates from Glass Cullet and Mining and Food Industry Carbonate Waste. Materials 2022, 15, 1223. https://doi.org/10.3390/ma15031223
Padilla I, López-Delgado A, Romero M. Glass Lightweight Aggregates from Glass Cullet and Mining and Food Industry Carbonate Waste. Materials. 2022; 15(3):1223. https://doi.org/10.3390/ma15031223
Chicago/Turabian StylePadilla, Isabel, Aurora López-Delgado, and Maximina Romero. 2022. "Glass Lightweight Aggregates from Glass Cullet and Mining and Food Industry Carbonate Waste" Materials 15, no. 3: 1223. https://doi.org/10.3390/ma15031223
APA StylePadilla, I., López-Delgado, A., & Romero, M. (2022). Glass Lightweight Aggregates from Glass Cullet and Mining and Food Industry Carbonate Waste. Materials, 15(3), 1223. https://doi.org/10.3390/ma15031223