Photoluminescence and Energy Transfer in Double- and Triple-Lanthanide-Doped YVO4 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structure and Morphology
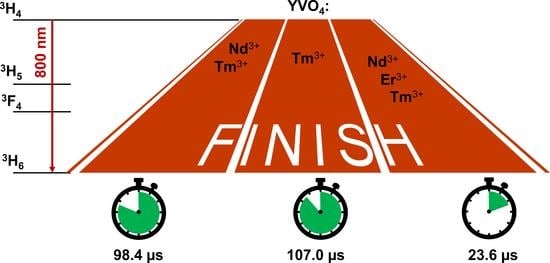
3.2. Luminescence Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cesaria, M.; Di Bartolo, B. Chapter 3 Nanophosphors: From Rare Earth Activated Multicolor-Tuning to New Efficient White Light Sources. In Quantum Nano-Photonics; Di Bartolo, B., Silvestri, L., Cesaria, M., Collins, J., Eds.; NATO Science for Peace and Security Series B: Physics and Biophysics; Springer: Dordrecht, The Netherlands, 2018; pp. 27–77. ISBN 978-94-024-1543-8. [Google Scholar]
- Zhu, Y.; Shen, X.; Zhou, M.; Su, X.; Li, J.; Yang, G.; Shao, H.; Zhou, Y. Ultra-Broadband 1.0 Μm Band Emission Spectroscopy in Pr3+/Nd3+/Yb3+ Tri-Doped Tellurite Glass. Spectrochim. Acta A 2019, 222, 117178. [Google Scholar] [CrossRef] [PubMed]
- Son, D.H.; Kim, B.H.; Lee, S.H.; Boo, S.; Han, W.-T. Ultra-Broadband near-Infrared Emission in Bismuth Borosilicate Glasses Incorporated with Er3+, Tm3+, and Yb3+ Ions. J. Non-Cryst. Solids 2014, 402, 106–110. [Google Scholar] [CrossRef]
- Zhao, D.; Yue, D.; Zhang, L.; Jiang, K.; Qian, G. Cryogenic Luminescent Tb/Eu-MOF Thermometer Based on a Fluorine-Modified Tetracarboxylate Ligand. Inorg. Chem. 2018, 57, 12596–12602. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, I.E.; Kurochkin, M.A.; Kalinichev, A.A.; Kolesnikov, E.Y.; Lähderanta, E. Optical Temperature Sensing in Tm3+/Yb3+-Doped GeO2–PbO–PbF2 Glass Ceramics Based on Ratiometric and Spectral Line Position Approaches. Sens. Actuator A Phys. 2018, 284, 251–259. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Afanaseva, E.V.; Kurochkin, M.A.; Vaishlia, E.I.; Kalinichev, A.A.; Kolesnikov, E.Y.; Lähderanta, E. Upconverting NIR-to-NIR LuVO 4:Nd 3+/Yb 3+ Nanophosphors for High-Sensitivity Optical Thermometry. ACS Appl. Mater. Interfaces 2022, 14, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhou, Z.; Shen, Y.; Zhou, Q.; Ji, J.; Liu, S.; Zhang, Y. Coupled Fluorometer-Potentiostat System and Metal-Free Monochromatic Luminophores for High-Resolution Wavelength-Resolved Electrochemiluminescent Multiplex Bioassay. ACS Sens. 2018, 3, 1362–1367. [Google Scholar] [CrossRef]
- Yu, S.; Tu, D.; Lian, W.; Xu, J.; Chen, X. Lanthanide-Doped near-Infrared II Luminescent Nanoprobes for Bioapplications. Sci. China Mater. 2019, 62, 1071–1086. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Zheng, W.; Gong, Z.; You, W.; Wei, J.; Chen, X. Rare Earth Ion- and Transition Metal Ion–Doped Inorganic Luminescent Nanocrystals: From Fundamentals to Biodetection. Mater. Today Nano 2019, 5, 100031. [Google Scholar] [CrossRef]
- Zan, G.; Wu, T.; Zhu, F.; He, P.; Cheng, Y.; Chai, S.; Wang, Y.; Huang, X.; Zhang, W.; Wan, Y.; et al. A Biomimetic Conductive Super-Foldable Material. Matter 2021, 4, 3232–3247. [Google Scholar] [CrossRef]
- Zhang, L.; Lyu, S.; Chen, Z.; Wang, S. Fabrication Flexible and Luminescent Nanofibrillated Cellulose Films with Modified SrAl2O4: Eu, Dy Phosphors via Nanoscale Silica and Aminosilane. Nanomaterials 2018, 8, 352. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Asanuma, T.; Hyodo, H.; Soga, K.; Matsumoto, M. Micromolding in Capillaries for Calcination-Free Fabrication of Flexible Inorganic Phosphor Films Consisting of Rare-Earth-Ion-Doped Nanoparticles. Langmuir 2013, 29, 11185–11191. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.L.; Szczurek, A.; Varas, S.; Armellini, C.; Scotognella, F.; Chiasera, A.; Ferrari, M.; Righini, G.C.; Lukowiak, A. Rare-Earth Activated SnO2 Photoluminescent Thin Films on Flexible Glass: Synthesis, Deposition and Characterization. Opt. Mater. 2022, 124, 111978. [Google Scholar] [CrossRef]
- Reddy, A.A.; Goel, A.; Tulyaganov, D.U.; Sardo, M.; Mafra, L.; Pascual, M.J.; Kharton, V.V.; Tsipis, E.V.; Kolotygin, V.A.; Ferreira, J.M.F. Thermal and Mechanical Stability of Lanthanide-Containing Glass–Ceramic Sealants for Solid Oxide Fuel Cells. J. Mater. Chem. A 2014, 2, 1834–1846. [Google Scholar] [CrossRef]
- Matovic, B.; Maletaskic, J.; Zagorac, J.; Pavkov, V.; Maki, R.S.S.; Yoshida, K.; Yano, T. Synthesis and Characterization of Pyrochlore Lanthanide (Pr, Sm) Zirconate Ceramics. J. Eur. Ceram. Soc. 2020, 40, 2652–2657. [Google Scholar] [CrossRef]
- Chen, L.; Guo, J.; Zhu, Y.; Hu, M.; Feng, J. Features of Crystal Structures and Thermo-mechanical Properties of Weberites RE3NbO7 (RE=La, Nd, Sm, Eu, Gd) Ceramics. J. Am. Ceram. Soc. 2021, 104, 404–412. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Wang, B.; Chang, X.; Yang, H.; Lan, J.; Wang, S.; Qiao, H.; Lin, H.; Han, S.; et al. A Functionalized Sm/Sr Doped TiO2 Nanotube Array on Titanium Implant Enables Exceptional Bone-Implant Integration and Also Self-Antibacterial Activity. Ceram. Int. 2020, 46, 14796–14807. [Google Scholar] [CrossRef]
- Jinga, S.-I.; Anghel, A.-M.; Brincoveanu, S.-F.; Bucur, R.-M.; Florea, A.-D.; Saftau, B.-I.; Stroe, S.-C.; Zamfirescu, A.-I.; Busuioc, C. Ce/Sm/Sr-Incorporating Ceramic Scaffolds Obtained via Sol-Gel Route. Materials 2021, 14, 1532. [Google Scholar] [CrossRef]
- Benali, A.; Azizi, S.; Bejar, M.; Dhahri, E.; Graça, M.F.P. Structural, Electrical and Ethanol Sensing Properties of Double-Doping LaFeO3 Perovskite Oxides. Ceram. Int. 2014, 40, 14367–14373. [Google Scholar] [CrossRef]
- Hossain, M.K.; Ahmed, M.H.; Khan, M.I.; Miah, M.S.; Hossain, S. Recent Progress of Rare Earth Oxides for Sensor, Detector, and Electronic Device Applications: A Review. ACS Appl. Electron. Mater. 2021, 3, 4255–4283. [Google Scholar] [CrossRef]
- Pei, P.; Wei, R.; Wang, B.; Su, J.; Zhang, Z.; Liu, W. An Advanced Tunable Multimodal Luminescent La4GeO8: Eu2+, Er3+ Phosphor for Multicolor Anticounterfeiting. Adv. Funct. Mater. 2021, 31, 2102479. [Google Scholar] [CrossRef]
- Pujales-Paradela, R.; Granath, T.; Seuffert, M.T.; Kasper, T.; Müller-Buschbaum, K.; Mandel, K. Luminescent Magnets: Hybrid Supraparticles of a Lanthanide-Based MOF and Ferromagnetic Iron Oxide by Assembly in a Droplet via Spray-Drying. J. Mater. Chem. C 2022, 10, 1017–1028. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Binyaseen, A.M.; Aljuhani, E.; Aljohani, M.; Alzahrani, H.K.; Shah, R.; El-Metwaly, N.M. Production of Smart Nanocomposite for Glass Coating toward Photochromic and Long-Persistent Photoluminescent Smart Windows. Ceram. Int. 2022, 48, 903–912. [Google Scholar] [CrossRef]
- Ghosh, K.; Murshed, M.M.; Frederichs, T.; Muniraju, N.K.C.; Gesing, T.M. Structural, Vibrational, Thermal, and Magnetic Properties of Mullite-type NdMnTiO5 Ceramic. J. Am. Ceram. Soc. 2022, 105, 2702–2712. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, Y.; Wang, R.; Li, X.; Fan, L.; Zhang, F. High-Capacity Upconversion Wavelength and Lifetime Binary Encoding for Multiplexed Biodetection. Angew. Chem. 2018, 130, 13006–13011. [Google Scholar] [CrossRef]
- Liu, H.; Jayakumar, M.K.G.; Huang, K.; Wang, Z.; Zheng, X.; Ågren, H.; Zhang, Y. Phase Angle Encoded Upconversion Luminescent Nanocrystals for Multiplexing Applications. Nanoscale 2017, 9, 1676–1686. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Haushalter, R.C.; Haushalter, R.W.; Shi, Y.; Zhang, Y.; Ding, K.; Zhao, D.; Stucky, G.D. Rare-Earth Upconverting Nanobarcodes for Multiplexed Biological Detection. Small 2011, 7, 1972–1976. [Google Scholar] [CrossRef] [Green Version]
- Gharouel, S.; Labrador-Páez, L.; Haro-González, P.; Horchani-Naifer, K.; Férid, M. Fluorescence Intensity Ratio and Lifetime Thermometry of Praseodymium Phosphates for Temperature Sensing. J. Lumin. 2018, 201, 372–383. [Google Scholar] [CrossRef]
- Kolesnikov, I.; Manshina, A. Rare Earth Ion Based Luminescence Thermometry. In Progress in Photon Science; Yamanouchi, K., Manshina, A.A., Makarov, V.A., Eds.; Springer Series in Chemical Physics; Springer: Cham, Switzerland, 2021; Volume 125, pp. 69–94. ISBN 978-3-030-77645-9. [Google Scholar]
- Medvedev, V.A.; Mamonova, D.V.; Kolesnikov, I.E.; Khokhlova, A.R.; Mikhailov, M.D.; Manshina, A.A. Synthesis and Luminescence Properties of YVO4: Nd3+, Er3+ and Tm3+ Nanoparticles. Inorg. Chem. Commun. 2020, 118, 107990. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Lvanova, T.Y.; Ivanov, D.A.; Kireev, A.A.; Mamonova, D.V.; Golyeva, E.V.; Mikhailov, M.D.; Manshina, A.A. In-Situ Laser-Induced Synthesis of Associated YVO4:Eu3+@SiO2@Au-Ag/C Nanohybrids with Enhanced Luminescence. J. Solid State Chem. 2018, 258, 835–840. [Google Scholar] [CrossRef]
- Singh, N.; Mugesh, G. CeVO4 Nanozymes Catalyze the Reduction of Dioxygen to Water without Releasing Partially Reduced Oxygen Species. Angew. Chem. 2019, 131, 7879–7883. [Google Scholar] [CrossRef]
- Paulose, P.I.; Jose, G.; Thomas, V.; Unnikrishnan, N.V.; Warrier, M.K.R. Sensitized Fluorescence of Ce3+/Mn2+ System in Phosphate Glass. J. Phys. Chem. Solids 2003, 64, 841–846. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Mamonova, D.V.; Kurochkin, M.A.; Kolesnikov, E.Y.; Lähderanta, E.; Manshina, A.A. YVO4 Nanoparticles Doped with Eu3+ and Nd3+ for Optical Nanothermometry. ACS Appl. Nano Mater. 2021, 4, 12481–12489. [Google Scholar] [CrossRef]








| № | Combinations of Rare Earth Ions | Concentration, at.% | ||
|---|---|---|---|---|
| Tm | Er | Nd | ||
| 1 | YVO4:Nd3+ | - | - | 0.03 |
| 2 | YVO4:Er3+ | - | 3.0 | - |
| 3 | YVO4:Tm3+ | 1.0 | - | - |
| 4 | YVO4:Tm3+, Er3+ | 1.0 | 3.0 | - |
| 5 | YVO4:Tm3+, Nd3+ | 1.0 | - | 0.03 |
| 6 | YVO4:Er3+, Nd3+ | - | 3.0 | 0.03 |
| 7 | YVO4:Tm3+, Er3+, Nd3+ | 1.0 | 3.0 | 0.03 |
| Combination of Er3+ Ions | |||
|---|---|---|---|
| Er3+, Tm3+, Nd3+ | Er3+, Tm3+ | Er3+, Nd3+ | |
| η, % | 17 | 10 | 0 |
| Combination of Tm3+ Ions | |||
|---|---|---|---|
| Er3+, Tm3+, Nd3+ | Er3+, Tm3+ | Tm3+, Nd3+ | |
| η, % | 78 | 78 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medvedev, V.A.; Kolesnikov, I.E.; Olshin, P.K.; Mikhailov, M.D.; Manshina, A.A.; Mamonova, D.V. Photoluminescence and Energy Transfer in Double- and Triple-Lanthanide-Doped YVO4 Nanoparticles. Materials 2022, 15, 2637. https://doi.org/10.3390/ma15072637
Medvedev VA, Kolesnikov IE, Olshin PK, Mikhailov MD, Manshina AA, Mamonova DV. Photoluminescence and Energy Transfer in Double- and Triple-Lanthanide-Doped YVO4 Nanoparticles. Materials. 2022; 15(7):2637. https://doi.org/10.3390/ma15072637
Chicago/Turabian StyleMedvedev, Vassiliy A., Ilya E. Kolesnikov, Pavel K. Olshin, Mikhail D. Mikhailov, Alina A. Manshina, and Daria V. Mamonova. 2022. "Photoluminescence and Energy Transfer in Double- and Triple-Lanthanide-Doped YVO4 Nanoparticles" Materials 15, no. 7: 2637. https://doi.org/10.3390/ma15072637
APA StyleMedvedev, V. A., Kolesnikov, I. E., Olshin, P. K., Mikhailov, M. D., Manshina, A. A., & Mamonova, D. V. (2022). Photoluminescence and Energy Transfer in Double- and Triple-Lanthanide-Doped YVO4 Nanoparticles. Materials, 15(7), 2637. https://doi.org/10.3390/ma15072637