Enhanced Photoelectrochemical Water Oxidation Using TiO2-Co3O4 p–n Heterostructures Derived from in Situ-Loaded ZIF-67
Abstract
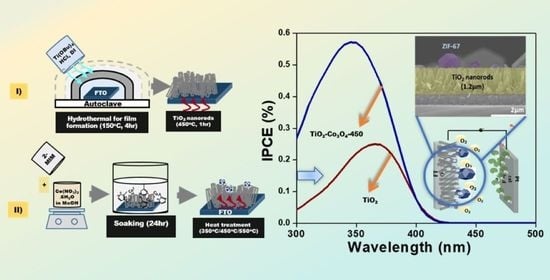
:1. Introduction
2. Experimental Section
2.1. Preparation of Rutile TiO2 Film (TiO2)
2.2. Preparation of TiO2-ZIF-67
2.3. Preparation of TiO2-Co3O4
2.4. Preparation of Photoanodes for PEC Tests
3. Results and Discussion
3.1. XRD Characterization of the Prepared Nanostructures
3.2. Morphology Characterization of the Prepared Nanostructures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Miquelot, A.; Debieu, O.; Rouessac, V.; Villeneuve, C.; Prud’Homme, N.; Cure, J.; Constantoudis, V.; Papavieros, G.; Roualdes, S.; Vahlas, C. TiO2 nanotree films for the production of green H2 by solar water splitting: From microstructural and optical characteristics to the photocatalytic properties. Appl. Surf. Sci. 2019, 494, 1127–1137. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Ali, S.L.; Salama, R.S. Promotional synergistic effect of Cs–Au NPs on the performance of Cs–Au/MgFe2O4 catalysts in catalysis 3,4-Dihydropyrimidin-2(1H)-Ones and degradation of RhB Dye. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3765–3776. [Google Scholar] [CrossRef]
- Liang, Z.; Hou, H.; Fang, Z.; Gao, F.; Wang, L.; Chen, D.; Yang, W. Hydrogenated TiO2 nanorod arrays decorated with carbon quantum dots toward efficient photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2019, 11, 19167–19175. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; You, S.; Du, J.; Dai, Y.; Chen, H.; Cai, Z.; Ren, N.; Zou, J. ZIF-67-derived Co3O4@ carbon protected by oxygen-buffering CeO2 as an efficient catalyst for boosting oxygen reduction/evolution reactions. J. Mater. Chem. A 2019, 7, 25853–25864. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Li, L.; Wang, J.; Zhang, Y.; Li, J.; Bai, J.; Xia, L.; Xu, Q.; Rahim, M. Carbon quantum dots modified anatase/rutile TiO2 photoanode with dramatically enhanced photoelectrochemical performance. Appl. Catal. B Environ. 2020, 269, 118776. [Google Scholar] [CrossRef]
- Wen, P.; Su, F.; Li, H.; Sun, Y.; Liang, Z.; Liang, W.; Zhang, J.; Qin, W.; Geyer, S.M.; Qiu, Y. A Ni2P nanocrystal cocatalyst enhanced TiO2 photoanode towards highly efficient photoelectrochemical water splitting. Chem. Eng. J. 2020, 385, 123878. [Google Scholar] [CrossRef]
- Cheng, X.; Dong, G.; Zhang, Y.; Feng, C.; Bi, Y. Dual-bonding interactions between MnO2 cocatalyst and TiO2 photoanodes for efficient solar water splitting. Appl. Catal. B Environ. 2020, 267, 118723. [Google Scholar] [CrossRef]
- Rui, K.; Zhao, G.; Chen, Y.; Lin, Y.; Zhou, Q.; Chen, J.; Zhu, J.; Sun, W.; Huang, W.; Dou, S.X. Hybrid 2D dual-metal–organic frameworks for enhanced water oxidation catalysis. Adv. Funct. Mater. 2018, 28, 1801554. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Dong, C.-L.; Zhou, W.; Lu, Y.-R.; Huang, Y.-C.; Liu, Y.; Guo, P.; Zhao, L.; Chou, W.-C.; Shen, S. A ternary nanostructured α-Fe2O3/Au/TiO2 photoanode with reconstructed interfaces for efficient photoelectrocatalytic water splitting. Appl. Catal. B Environ. 2020, 260, 118206. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, B.; Sang, Y.; Liu, H. Heterostructures construction on TiO2 nanobelts: A powerful tool for building high-performance photocatalysts. Appl. Catal. B Environ. 2017, 202, 620–641. [Google Scholar] [CrossRef]
- Pan, K.; Dong, Y.; Zhou, W.; Pan, Q.; Xie, Y.; Xie, T.; Tian, G.; Wang, G. Facile fabrication of hierarchical TiO2 nanobelt/ZnO nanorod heterogeneous nanostructure: An efficient photoanode for water splitting. ACS Appl. Mater. Interfaces 2013, 5, 8314–8320. [Google Scholar] [CrossRef]
- Qu, F.; Jiang, H.; Yang, M. MOF-derived Co3O4/NiCo2O4 double-shelled nanocages with excellent gas sensing properties. Mater. Lett. 2017, 190, 75–78. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Sun, F.; Qi, J.; Zhang, D.; Sun, H.; Li, Z.; Wang, Q.; Wang, B. Construction of Co3O4/CeO2 heterostructure nanoflowers facilitates deployment of oxygen defects to enhance the oxygen evolution kinetics. J. Alloys Compd. 2023, 933, 167700. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, D.; Sun, H.; Wang, Q.; Li, Z.; Qi, J.; Wang, B. Confinement amorphous cobalt-nickel oxide polyhedral yolk-shell structures for enhanced oxygen evolution performance. Appl. Surf. Sci. 2023, 613, 156088. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Zuo, G.; Guo, Y.; Xiao, W.; Dai, Y.; Kong, J.; Xu, X.; Zhou, Y.; Xie, A. 0D/2D Co3O4/TiO2 Z-Scheme heterojunction for boosted photocatalytic degradation and mechanism investigation. Appl. Catal. B Environ. 2020, 278, 119298. [Google Scholar] [CrossRef]
- Liu, J.; Ke, J.; Li, Y.; Liu, B.; Wang, L.; Xiao, H.; Wang, S. Co3O4 quantum dots/TiO2 nanobelt hybrids for highly efficient photocatalytic overall water splitting. Appl. Catal. B Environ. 2018, 236, 396–403. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, L.; Liu, X.; Wang, Y.; Xing, S. Enhancing the Catalytic Activity of Zeolitic Imidazolate Framework-8-Derived N-Doped Carbon with Incorporated CeO2 Nanoparticles in the Oxygen Reduction Reaction. Chem.–A Eur. J. 2017, 23, 10690–10697. [Google Scholar] [CrossRef]
- He, D.; Wu, X.; Liu, W.; Lei, C.; Yu, C.; Zheng, G.; Pan, J.; Lei, L.; Zhang, X. Co1-xS embedded in porous carbon derived from metal organic framework as a highly efficient electrocatalyst for oxygen evolution reaction. Chin. Chem. Lett. 2019, 30, 229–233. [Google Scholar] [CrossRef]
- Wu, H.B.; Lou, X.W. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Sci. Adv. 2017, 3, eaap9252. [Google Scholar] [CrossRef] [Green Version]
- Salunkhe, R.R.; Tang, J.; Kamachi, Y.; Nakato, T.; Kim, J.H.; Yamauchi, Y. Asymmetric supercapacitors using 3D nanoporous carbon and cobalt oxide electrodes synthesized from a single metal–organic framework. ACS Nano 2015, 9, 6288–6296. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, F.; Xia, Y.; Li, J.; Tamirat, A.G.; Liu, Y.; Wang, L.; Wang, Y.; Xia, Y. In situ encapsulation of core–shell-structured Co@ Co3O4 into nitrogen-doped carbon polyhedra as a bifunctional catalyst for rechargeable Zn–air batteries. J. Mater. Chem. A 2018, 6, 1443–1453. [Google Scholar] [CrossRef]
- Xu, H.; Cao, J.; Shan, C.; Wang, B.; Xi, P.; Liu, W.; Tang, Y. MOF-derived hollow CoS decorated with CeOx nanoparticles for boosting oxygen evolution reaction electrocatalysis. Angew. Chem. 2018, 130, 8790–8794. [Google Scholar] [CrossRef]
- Wang, M.-X.; Zhang, J.; Fan, H.-L.; Liu, B.-X.; Yi, X.-B.; Wang, J.-Q. ZIF-67 derived Co3O4/carbon aerogel composite for supercapacitor electrodes. New J. Chem. 2019, 43, 5666–5669. [Google Scholar] [CrossRef]
- Du, N.; Wang, C.; Long, R.; Xiong, Y. N-doped carbon-stabilized PtCo nanoparticles derived from Pt@ ZIF-67: Highly active and durable catalysts for oxygen reduction reaction. Nano Res. 2017, 10, 3228–3237. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, S.; Xu, L.; Wang, D.; Li, Y. An efficient multifunctional hybrid electrocatalyst: Ni2P nanoparticles on MOF-derived Co, N-doped porous carbon polyhedrons for oxygen reduction and water splitting. Chem. Commun. 2018, 54, 12101–12104. [Google Scholar] [CrossRef]
- Ding, Q.; Gou, L.; Wei, D.; Xu, D.; Fan, W.; Shi, W. Metal-organic framework derived Co3O4/TiO2 heterostructure nanoarrays for promote photoelectrochemical water splitting. Int. J. Hydrogen Energy 2021, 46, 24965–24976. [Google Scholar] [CrossRef]
- Guo, Z.; Wei, J.; Zhang, B.; Ruan, M.; Liu, Z. Construction and photoelectrocatalytic performance of TiO2/BiVO4 heterojunction modified with cobalt phosphate. J. Alloys Compd. 2020, 821, 153225. [Google Scholar] [CrossRef]
- Goud, B.S.; Shin, G.; Vattikuti, S.P.; Mameda, N.; Kim, H.; Koyyada, G.; Kim, J.H. Enzyme-integrated biomimetic cobalt metal-organic framework nanozyme for one-step cascade glucose biosensing via tandem catalysis. Biochem. Eng. J. 2022, 188, 108669. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Wang, Z.; Huang, H.; Peng, H.; Li, X. Fabrication of mesoporous Co3O4 oxides by acid treatment and their catalytic performances for toluene oxidation. Appl. Catal. A Gen. 2018, 550, 67–76. [Google Scholar] [CrossRef]
- Thanh Thuy, C.T.; Shin, G.; Jieun, L.; Kim, H.D.; Koyyada, G.; Kim, J.H. Self-doped carbon dots decorated TiO2 nanorods: A novel synthesis route for enhanced photoelectrochemical water splitting. Catalysts 2022, 12, 1281. [Google Scholar] [CrossRef]
- Wei, N.; Liu, Y.; Feng, M.; Li, Z.; Chen, S.; Zheng, Y.; Wang, D. Controllable TiO2 core-shell phase heterojunction for efficient photoelectrochemical water splitting under solar light. Appl. Catal. B Environ. 2019, 244, 519–528. [Google Scholar] [CrossRef]
- Li, J.; Lu, G.; Wu, G.; Mao, D.; Guo, Y.; Wang, Y.; Guo, Y. Effect of TiO2 crystal structure on the catalytic performance of Co3O4/TiO2 catalyst for low-temperature CO oxidation. Catal. Sci. Technol. 2014, 4, 1268–1275. [Google Scholar] [CrossRef]
- Gao, L.; Gan, W.; Qiu, Z.; Zhan, X.; Qiang, T.; Li, J. Preparation of heterostructured WO3/TiO2 catalysts from wood fibers and its versatile photodegradation abilities. Sci. Rep. 2017, 7, 1102. [Google Scholar] [CrossRef] [Green Version]
- Zhu, R.; Ding, J.; Yang, J.; Pang, H.; Xu, Q.; Zhang, D.; Braunstein, P. Quasi-ZIF-67 for boosted oxygen evolution reaction catalytic activity via a low temperature calcination. ACS Appl. Mater. Interfaces 2020, 12, 25037–25041. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Ding, J.; Xu, Y.; Yang, J.; Xu, Q.; Pang, H. π-Conjugated molecule boosts metal–organic frameworks as efficient oxygen evolution reaction catalysts. Small 2018, 14, 1803576. [Google Scholar] [CrossRef]
- Wang, L.; Qi, T.; Wang, J.; Zhang, S.; Xiao, H.; Ma, Y. Uniform dispersion of cobalt nanoparticles over nonporous TiO2 with low activation energy for magnesium sulfate recovery in a novel magnesia-based desulfurization process. J. Hazard. Mater. 2018, 342, 579–588. [Google Scholar] [CrossRef]
- Liu, N.; Tang, M.; Jing, C.; Huang, W.; Tao, P.; Zhang, X.; Lei, J.; Tang, L. Synthesis of highly efficient Co3O4 catalysts by heat treatment ZIF-67 for CO oxidation. J. Sol-Gel Sci. Technol. 2018, 88, 163–171. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Ai, T.; Bao, W.; Dong, H.; Jiang, P.; Zou, X. An efficient hydrogen evolution by self-supported nickel sulfur-based hybrid nanoplate electrocatalyst. Ionics 2022, 28, 353–360. [Google Scholar] [CrossRef]
- Mishra, I.K.; Zhou, H.; Sun, J.; Dahal, K.; Ren, Z.; He, R.; Chen, S.; Ren, Z. Highly efficient hydrogen evolution by self-standing nickel phosphide-based hybrid nanosheet arrays electrocatalyst. Mater. Today Phys. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Wang, T.; Long, X.; Wei, S.; Wang, P.; Wang, C.; Jin, J.; Hu, G. Boosting hole transfer in the fluorine-doped hematite photoanode by depositing ultrathin amorphous FeOOH/CoOOH cocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 49705–49712. [Google Scholar] [CrossRef]
- Han, Y.; Wu, J.; Li, Y.; Gu, X.; He, T.; Zhao, Y.; Huang, H.; Liu, Y.; Kang, Z. Carbon dots enhance the interface electron transfer and photoelectrochemical kinetics in TiO2 photoanode. Appl. Catal. B Environ. 2022, 304, 120983. [Google Scholar]
- Bai, S.; Liu, J.; Cui, M.; Luo, R.; He, J.; Chen, A. Two-step electrodeposition to fabricate the p–n heterojunction of a Cu2O/BiVO4 photoanode for the enhancement of photoelectrochemical water splitting. Dalton Trans. 2018, 47, 6763–6771. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yang, C.; Cheng, H.; Jiao, S.; Takeda, O.; Zhu, H. High-performance p-Cu2O/n-TaON heterojunction nanorod photoanodes passivated with an ultrathin carbon sheath for photoelectrochemical water splitting. Energy Environ. Sci. 2014, 7, 3758–3768. [Google Scholar]
- Afroz, K.; Moniruddin, M.; Bakranov, N.; Kudaibergenov, S.; Nuraje, N. A heterojunction strategy to improve the visible light sensitive water splitting performance of photocatalytic materials. J. Mater. Chem. A 2018, 6, 21696–21718. [Google Scholar]
- Dasineh Khiavi, N.; Katal, R.; Kholghi Eshkalak, S.; Masudy-Panah, S.; Ramakrishna, S.; Jiangyong, H. Visible light driven heterojunction photocatalyst of CuO–Cu2O thin films for photocatalytic degradation of organic pollutants. Nanomaterials 2019, 9, 1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Yan, L. High-efficiency p–n junction oxide photoelectrodes for photoelectrochemical water splitting. Phys. Chem. Chem. Phys. 2016, 18, 31230–31237. [Google Scholar] [CrossRef]
- Cui, W.; Shang, J.; Bai, H.; Hu, J.; Xu, D.; Ding, J.; Fan, W.; Shi, W. In-situ implantation of plasmonic Ag into metal-organic frameworks for constructing efficient Ag/NH2-MIL-125/TiO2 photoanode. Chem. Eng. J. 2020, 388, 124206. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Liu, C.; Liang, P.; Mitsuzaki, N.; Chen, Z. Effect of Co-Based Metal–Organic Framework Prepared by an In Situ Growth Method on the Photoelectrochemical Performance of Electrodeposited Hematite Photoanode. Energy Technol. 2019, 7, 1801069. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Zhang, P.; Zhang, J.; Li, A.; Gong, J. Enhanced Surface Reaction Kinetics and Charge Separation of p–n Heterojunction Co3O4/BiVO4 Photoanodes. J. Am. Chem. Soc. 2015, 137, 8356–8359. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Y.; Qu, J.; Zheng, R.; Cairney, J.M.; Zhang, J.; Zhu, M.; Khan, A.; Li, W. Enhanced photoelectrochemical water-splitting performance with a hierarchical heterostructure: Co3O4 nanodots anchored TiO2@P-C3N4 core-shell nanorod arrays. Chem. Eng. J. 2021, 404, 126458. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, K.; Huang, J.; Wang, L.; Zhang, M.; Bai, B.; Liu, H.; Wang, Q. Preparation of heterometallic CoNi-MOFs-modified BiVO4: A steady photoanode for improved performance in photoelectrochemical water splitting. Appl. Catal. B Environ. 2020, 266, 118513. [Google Scholar]
- Cui, W.; Bai, H.; Shang, J.; Wang, F.; Xu, D.; Ding, J.; Fan, W.; Shi, W. Organic-inorganic hybrid-photoanode built from NiFe-MOF and TiO2 for efficient PEC water splitting. Electrochim. Acta 2020, 349, 136383. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanh Thu, C.T.; Jo, H.J.; Koyyada, G.; Kim, D.-H.; Kim, J.H. Enhanced Photoelectrochemical Water Oxidation Using TiO2-Co3O4 p–n Heterostructures Derived from in Situ-Loaded ZIF-67. Materials 2023, 16, 5461. https://doi.org/10.3390/ma16155461
Thanh Thu CT, Jo HJ, Koyyada G, Kim D-H, Kim JH. Enhanced Photoelectrochemical Water Oxidation Using TiO2-Co3O4 p–n Heterostructures Derived from in Situ-Loaded ZIF-67. Materials. 2023; 16(15):5461. https://doi.org/10.3390/ma16155461
Chicago/Turabian StyleThanh Thu, Chau Thi, Hyo Jeong Jo, Ganesh Koyyada, Dae-Hwan Kim, and Jae Hong Kim. 2023. "Enhanced Photoelectrochemical Water Oxidation Using TiO2-Co3O4 p–n Heterostructures Derived from in Situ-Loaded ZIF-67" Materials 16, no. 15: 5461. https://doi.org/10.3390/ma16155461
APA StyleThanh Thu, C. T., Jo, H. J., Koyyada, G., Kim, D. -H., & Kim, J. H. (2023). Enhanced Photoelectrochemical Water Oxidation Using TiO2-Co3O4 p–n Heterostructures Derived from in Situ-Loaded ZIF-67. Materials, 16(15), 5461. https://doi.org/10.3390/ma16155461