Optimization of Chitosan Extraction Process from Rapana venosa Egg Capsules Waste Using Experimental Design
Abstract
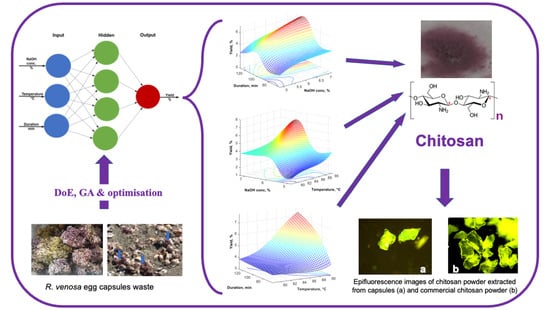
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chitosan Extraction Procedure
2.3. Analytical Methods
2.3.1. Microscopic Analysis
2.3.2. Determination of Deacetylation Degree
2.3.3. Molar Mass
3. Results and Discussion
3.1. Optimization of the Extraction Process
3.2. Optimizing the Extraction Yield Using Neural Networks
3.3. Chitosan Characterization
3.3.1. Fluorescence Microscopy
3.3.2. Deacetylation Degree (DD) and Molar Mass (MW)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ioelovich, M. Crystallinity and Hydrophility of Chitin and Chitosan. Res. Rev. J. Chem. 2014, 3, 7–14. [Google Scholar]
- Knežević-Jugović, Z.D.; Bezbradica, D.I.; Mijin, D.Ž.; Antov, M.G. The Immobilization of Enzyme on Eupergit® Supports by Covalent Attachment. In Enzyme Stabilization and Immobilization. Methods in Molecular Biology; Minteer, S., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 99–111. [Google Scholar]
- Bakshia, P.S.; Selvakumara, D.; Kadirvelub, K.; Kumara, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Blagodatskikh, I.V.; Kulikov, S.N.; Vyshivannaya, O.V.; Bezrodnykh, E.A.; Yamskov, I.A.; Tikhonov, V.E. Influence of glucosamine on oligochitosan solubility and antibacterial activity. Carbohydr. Res. 2013, 381, 28–32. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.-W.; Kim, S.-K.; Jeon, Y.-J.; Je, J.-Y.; Ahn, C.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Chitooligosaccharide and Its Derivatives: Preparation and Biological Applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [Green Version]
- Mourya, V.K.; Inamdar, N.N.; Choudhari, Y.M. Chitooligosaccharides: Synthesis, characterization and applications. Polym. Sci. Ser. A 2011, 53, 583–612. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Varum, K.M.; Eijsink, V.G.H. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, G.-L.; Zhu, M.-J. Preparation of astaxanthin-encapsulated complex with zein and oligochitosan and its application in food processing. LWT 2019, 106, 179–185. [Google Scholar] [CrossRef]
- Green, J.H.; Mattick, J.F. Fishery waste management. In Food Processing Waste Management; Green, J.H., Kramer, A., Eds.; AVI Publishing: Westport, UK, 1979; pp. 202–307. [Google Scholar]
- Subhapradha, N.; Ramasamy, P.; Shanmugam, V.; Madeswaran, P.; Srinivasan, A.; Shanmugam, A. Physicochemical characterisation of β-chitosan from Sepioteuthis lessoniana gladius. Food Chem. 2013, 141, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Apetroaei, M.R.; Zgârian, R.G.; Manea, A.M.; Rău, I.; Tihan, G.T.; Schröder, V. New source of chitosan from Black Sea marine organisms identification. Mol. Cryst. Liq. Cryst. 2016, 628, 102–109. [Google Scholar] [CrossRef]
- Lee, J.W.; Yeomans, W.G.; Allen, A.L.; Deng, F.; Gross, R.A.; Kaplan, D.L. Production of Chitosan-and Chitin-Like Exopolymers, Patent No. US6534294B1, 18 March 2003. [Google Scholar]
- Chatterjee, S.; Adhya, M.; Guha, A.K.; Chatterjee, B.P. Chitosan from Mucor rouxii: Production and physico-chemical characterization. Process Biochem. 2005, 40, 395–400. [Google Scholar] [CrossRef]
- Schröder, V.; Rău, I.; Dobrin, N.; Stefanov, C.; Mihali, C.-V.; Pădureţu, C.-C.; Apetroaei, M.R. Micromorphological details and identification of chitinous wall structures in Rapana venosa (Gastropoda, Mollusca) egg capsules. Sci. Rep. 2020, 10, 14550. [Google Scholar] [CrossRef] [PubMed]
- Pădurețu, C.C.; Isopescu, R.; Rău, I.; Apetroaei, M.R.; Schröder, V. Influence of the parameters of chitin deacetylation process on the chitosan obtained from crab shell waste. Korean J. Chem. Eng. 2019, 36, 1890–1899. [Google Scholar] [CrossRef]
- Bello, V.E.; Olafadeha, O.A. Comparative investigation of RSM and ANN for multi-response modeling and optimization studies of derived chitosan from Archachatina marginate shell. Alex. Eng. J. 2021, 60, 3869–3899. [Google Scholar] [CrossRef]
- Abdel-Gawad, K.M.; Hifney, A.F.; Fawzy, M.A.; Gomaa, M. Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll. 2017, 63, 593–601. [Google Scholar] [CrossRef]
- Dima, J.B.; Sequeiros, C.; Zaritzky, N. Chitosan from Marine Crustaceans: Production, Characterization and Applications. In Biological Activities and Application of Marine Polysaccharides, 1st ed.; Shalaby, E.A., Ed.; InTech Open: London, UK, 2017; pp. 39–56. [Google Scholar]
- Roshchina, V.V. Vital Autofluorescence: Application to the study of plant living cells. Int. J. Spectrosc. 2012, 2012, 124672. [Google Scholar] [CrossRef]







| Exp. No. | NaOH Conc. (%) | Temperature (°C) | Yield (%) | Observations Deproteinization Time = 120 min megg:vol NaOH = 1/40 |
|---|---|---|---|---|
| 1 | 4 | 80 | 1.72 | At the end, about 50% egg capsules did not dissolve |
| 2 | 6 | 80 | 4.16 | At the end, part of the capsules remained undissolved and were found in the centrifuged filtrate |
| 3 | 4 | 90 | 4.76 | At the end, all the capsules were dissolved |
| 4 | 6 | 90 | 5.20 | At the end, all the capsules were dissolved |
| 5 | 5 | 85 | 3.00 | At the end, when filtering the obtained suspension, a small number of capsules remained in the form of gelatine (partially dissolved) |
| 6 | 5 | 85 | 2.00 | |
| 7 | 5 | 85 | 2.00 |
| Duration (min.) | 60 | 85 | |
|---|---|---|---|
| NaOH Conc., (%) | |||
| 5 | 1.96 | 3.04 | |
| 1.84 | 3.20 | ||
| 6 | 3.32 | 4.92 | |
| 3.28 | 3.92 | ||
| 7 | 2.88 | 4.00 | |
| 3.12 | 4.92 |
| Exp. No. | NaOH Conc. (%) | Temperature (°C) | Duration (min) | Yield (%) | |
|---|---|---|---|---|---|
| Estimated by the Preliminary Model | Experimental | ||||
| 1 | 7 | 85 | 120 | 4.15 | 4.13 |
| 2 | 6.5 | 90 | 120 | 6.18 | 7.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinculescu, D.; Gîjiu, C.L.; Apetroaei, M.R.; Isopescu, R.; Rău, I.; Schröder, V. Optimization of Chitosan Extraction Process from Rapana venosa Egg Capsules Waste Using Experimental Design. Materials 2023, 16, 525. https://doi.org/10.3390/ma16020525
Dinculescu D, Gîjiu CL, Apetroaei MR, Isopescu R, Rău I, Schröder V. Optimization of Chitosan Extraction Process from Rapana venosa Egg Capsules Waste Using Experimental Design. Materials. 2023; 16(2):525. https://doi.org/10.3390/ma16020525
Chicago/Turabian StyleDinculescu, Daniel, Cristiana Luminița Gîjiu, Manuela Rossemary Apetroaei, Raluca Isopescu, Ileana Rău, and Verginica Schröder. 2023. "Optimization of Chitosan Extraction Process from Rapana venosa Egg Capsules Waste Using Experimental Design" Materials 16, no. 2: 525. https://doi.org/10.3390/ma16020525
APA StyleDinculescu, D., Gîjiu, C. L., Apetroaei, M. R., Isopescu, R., Rău, I., & Schröder, V. (2023). Optimization of Chitosan Extraction Process from Rapana venosa Egg Capsules Waste Using Experimental Design. Materials, 16(2), 525. https://doi.org/10.3390/ma16020525