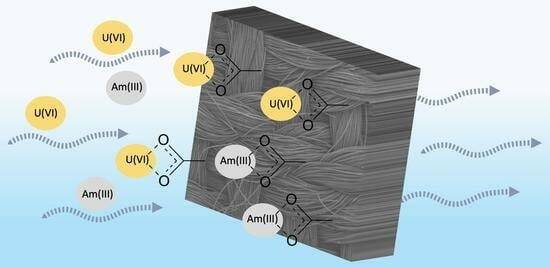
Radionuclide Removal from Aqueous Solutions Using Oxidized Carbon Fabrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Carbon Fabric Oxidation and Characterization
2.2.2. Adsorption Experiments
3. Results and Discussion
3.1. Carbon Fabric Characterization
3.2. Radionuclide Adsorption
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Thermodynamics
3.2.3. Application to Seawater Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inglezakis, V.J.; Poulopoulos, S.G.; Arkhangelsky, E.; Zorpas, A.A.; Menegaki, A.N. Aquatic Environment. In Environment and Development; Elsevier: Amsterdam, The Netherlands, 2016; pp. 137–212. [Google Scholar]
- Lariviere, D.; Taylor, V.F.; Evans, R.D.; Cornett, R.J. Radionuclide Determination in Environmental Samples by Inductively Coupled Plasma Mass Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 877–904. [Google Scholar] [CrossRef]
- Xu, M. Syntheses, Structures, and Solution Behavior of Organic-Functionalized Uranyl Peroxide Nanoclusters; University of Notre Dame: Notre Dame, IN, USA, 2021. [Google Scholar]
- Ewing, R.C. Ceramic Matrices for Plutonium Disposition. Prog. Nucl. Energy 2007, 49, 635–643. [Google Scholar] [CrossRef]
- Humphrey, U.E.; Khandaker, M.U. Viability of Thorium-Based Nuclear Fuel Cycle for the Next Generation Nuclear Reactor: Issues and Prospects. Renew. Sustain. Energy Rev. 2018, 97, 259–275. [Google Scholar] [CrossRef]
- Ioannidis, I.; Kinigopoulou, V.; Giannakoudakis, D.A.; Arkas, M.; Anastopoulos, I.; Triantafyllidis, K.S.; Pashalidis, I. Microplastics and Disposable Face Masks as “Trojan Horse” for Radionuclides Pollution in Water Bodies—A Review with Emphasis on the Involved Interactions. Sustain. Chem. Environ. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Kouzes, R.T.; Siciliano, E.R.; Ely, J.H.; Keller, P.E.; McConn, R.J. Passive Neutron Detection for Interdiction of Nuclear Material at Borders. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2008, 584, 383–400. [Google Scholar] [CrossRef]
- Osváth, S.; Vajda, N.; Molnár, Z. Development of a Complex Method for the Determination of Actinides. J. Radioanal. Nucl. Chem. 2009, 281, 461–465. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, X.; Chen, H.; Zhang, L.; Li, J.; Guo, Y.; Sun, Z. Biomineralization of Uranium by Desulfovibrio desulfuricans A3-21ZLL under Various Hydrochemical Conditions. Environ. Res. 2023, 237, 116950. [Google Scholar] [CrossRef]
- Georgiou, E.; Raptopoulos, G.; Anastopoulos, I.; Giannakoudakis, D.A.; Arkas, M.; Paraskevopoulou, P.; Pashalidis, I. Uranium Removal from Aqueous Solutions by Aerogel-Based Adsorbents—A Critical Review. Nanomaterials 2023, 13, 363. [Google Scholar] [CrossRef]
- Collins, R.N.; Saito, T.; Aoyagi, N.; Payne, T.E.; Kimura, T.; Waite, T.D. Applications of Time-Resolved Laser Fluorescence Spectroscopy to the Environmental Biogeochemistry of Actinides. J. Environ. Qual. 2011, 40, 731–741. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A. The Use of Rare Earth Element Profiles as a Proxy for Fractionation Source and Mine-Waste Provenance. Sci. Total Environ. 2023, 901, 166517. [Google Scholar] [CrossRef]
- Debruyne, D.; Hulsbosch, N.; Muchez, P. Unraveling Rare Earth Element Signatures in Hydrothermal Carbonate Minerals Using a Source–Sink System. Ore Geol. Rev. 2016, 72, 232–252. [Google Scholar] [CrossRef]
- Wang, J.; Shao, L.; Wang, X. The Coal-Forming Environment at the End of the Late Permian and Its Control on Trace Elements: The Upper Xuanwei Formation in Eastern Yunnan, China. Processes 2023, 11, 2936. [Google Scholar] [CrossRef]
- Meinrath, G.; Volke, P.; Helling, C.; Dudel, E.G.; Merkel, B.J. Determination and Interpretation of Environmental Water Samples Contaminated by Uranium Mining Activities. Fresenius’ J. Anal. Chem. 1999, 364, 191–202. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Dong, X.; Wu, T.; Qing, Q.; Chen, J.; Lu, Y.; Xu, C. Ion Sieving in Graphene Oxide Membrane Enables Efficient Actinides/Lanthanides Separation. Nat. Commun. 2023, 14, 261. [Google Scholar] [CrossRef] [PubMed]
- Nash, K.L. Separation Chemistry for Lanthanides and Trivalent Actinides. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 1994; Volume 18, pp. 197–238. [Google Scholar]
- Seo, S.D.; Truong-Lam, H.S.; Jeon, C.; Han, J.; Kang, K.; Lee, S.; Lee, J.D. Simultaneous Removal of Multi-Nuclide (Sr2+, Co2+, Cs+, and I−) from Aquatic Environments Using a Hydrate-Based Water Purification Process. J. Hazard. Mater. 2023, 462, 132700. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.A. Physical, Chemical, and Biological Treatment of Groundwater at Contaminated Nuclear and NORM Sites. In Environmental Remediation and Restoration of Contaminated Nuclear and NORM Sites; Woodhead Publishing: Cambridge, UK, 2015; pp. 237–256. [Google Scholar]
- Khedr, M.G. Radioactive Contamination of Groundwater: Special Aspects and Advantages of Removal by Reverse Osmosis and Nanofiltration. Desalination 2013, 321, 47–54. [Google Scholar] [CrossRef]
- Gäfvert, T.; Ellmark, C.; Holm, E. Removal of Radionuclides at a Waterworks. J. Environ. Radioact. 2002, 63, 105–115. [Google Scholar] [CrossRef]
- Kucserka, T.; Németh, G.I.; Pálfi, I.; Kiss, Z.L.; Tombácz, E.; Galambos, I. Adsorption-Based Pretreatment of Irrigation Water to Prevent Water Quality Issues. Separations 2023, 10, 468. [Google Scholar] [CrossRef]
- Habineza, A.; Zhai, J.; Ntakirutimana, T.; Qiu, F.P.; Li, X.; Wang, Q. Heavy Metal Removal from Wastewaters by Agricultural Waste Low-Cost Adsorbents: Hindrances of Adsorption Technology to the Large Scale Industrial Application—A Review. Desalin. Water Treat. 2017, 78, 192–214. [Google Scholar] [CrossRef]
- Ibrahim, A.O.; Adegoke, K.A.; Adegoke, R.O.; AbdulWahab, Y.A.; Oyelami, V.B.; Adesina, M.O. Adsorptive removal of different pollutants using metal-organic framework adsorbents. J. Mol. Liq. 2021, 333, 115593. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Ray, S.S. Recent Advances in Carbon Nanomaterial-Based Adsorbents for Water Purification. Coord. Chem. Rev. 2020, 405, 213111. [Google Scholar]
- Kostoglou, N.; Koczwara, C.; Prehal, C.; Terziyska, V.; Babic, B.; Matovic, B.; Constantinides, G.; Tampaxis, C.; Charalambopoulou, G.; Steriotis, T.; et al. Nanoporous Activated Carbon Cloth as a Versatile Material for Hydrogen Adsorption, Selective Gas Separation, and Electrochemical Energy Storage. Nano Energy 2017, 40, 49–64. [Google Scholar] [CrossRef]
- Pendolino, F.; Armata, N. Graphene Oxide in Environmental Remediation Process; Springer: Cham, Switzerland, 2017; Volume 7, p. 11. [Google Scholar]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the Future of Membranes: Perspectives for Advanced and New Membrane Materials and Manufacturing Processes. J. Membr. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- Jia, R.; Sun, D.; Dang, Y.; Meier, D.; Holmes, D.E.; Smith, J.A. Carbon Cloth Enhances Treatment of High-Strength Brewery Wastewater in Anaerobic Dynamic Membrane Bioreactors. Bioresour. Technol. 2020, 298, 122547. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Delgado, C.; Partida-Gutierrez, D.; Rangel-Mendez, J.R. Preparation of activated carbon cloths from renewable natural fabrics and their performance during the adsorption of model organic and inorganic pollutants in water. J. Clean. Prod. 2019, 213, 650–658. [Google Scholar] [CrossRef]
- Guedidi, H.; Reinert, L.; Sonde, Y.; Bellakhal, N.; Duclaux, L. Adsorption of ibuprofen from aqueous solution on chemically surface-modified activated carbon cloths. Arab. J. Chem. 2017, 10, S3584–S3594. [Google Scholar] [CrossRef]
- Masson, S.; Gineys, M.; Delpeux-Ouldriane, S.; Reinert, L.; Guittonneau, S.; Béguin, F.; Duclaux, L. Single, binary, and mixture adsorption of nine organic contaminants onto a microporous and a microporous/mesoporous activated carbon cloth. Microporous Mesoporous Mater. 2016, 234, 24–34. [Google Scholar] [CrossRef]
- Kim, C.; Srimuk, P.; Lee, J.; Fleischmann, S.; Aslan, M.; Presser, V. Influence of pore structure and cell voltage of activated carbon cloth as a versatile electrode material for capacitive deionization. Carbon 2017, 122, 329–335. [Google Scholar] [CrossRef]
- Alkhaldi, T.; Blankenship, L.S.; Mokaya, R. A simple, sustainable route to flexible microporous carbon cloth for energy storage applications. Mater. Adv. 2023, 4, 3559–3571. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B.; Gao, Y.; Zhang, J.; Wang, B. Optimization performance of viscose rayon derived flexible porous carbon cloths in relation to their CO2 capture, CO2/CH4 separation and methanol adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2023, 677, 132350. [Google Scholar] [CrossRef]
- Attia, N.F.; Jung, M.; Park, J.; Jang, H.; Lee, K.; Oh, H. Flexible nanoporous activated carbon cloth for achieving high H2, CH4, and CO2 storage capacities and selective CO2/CH4 separation. Chem. Eng. J. 2020, 379, 122367. [Google Scholar] [CrossRef]
- Florent, M.; Giannakoudakis, D.A.; Bandosz, T.J. Mustard gas surrogate interactions with modified porous carbon fabrics: Effect of oxidative treatment. Langmuir 2017, 33, 11475–11483. [Google Scholar] [CrossRef] [PubMed]
- Giannakoudakis, D.A.; Barczak, M.; Florent, M.; Bandosz, T.J. Analysis of interactions of mustard gas surrogate vapors with porous carbon textiles. Chem. Eng. J. 2019, 362, 758–766. [Google Scholar] [CrossRef]
- Jung, M.; Park, J.; Cho, S.Y.; Elashery, S.A.E.; Attia, N.F.; Oh, H. Flexible carbon sieve based on nanoporous carbon cloth for efficient CO2/CH4 separation. Surf. Interfaces 2021, 23, 100960. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Barczak, M.; Pearsall, F.; O’Brien, S.; Bandosz, T.J. Composite porous carbon textile with deposited barium titanate nanospheres as wearable protection medium against toxic vapors. Chem. Eng. J. 2020, 384, 123280. [Google Scholar] [CrossRef]
- Wallace, R.; Giannakoudakis, D.A.; Florent, M.; Karwacki, C.J.; Bandosz, T. Ferrihydrite deposited on cotton textiles as protection media against the chemical warfare agent surrogate (2-chloroethyl ethyl sulfide). J. Mater. Chem. A 2017, 5, 4972–4981. [Google Scholar] [CrossRef]
- Florent, M.; Giannakoudakis, D.A.; Wallace, R.; Bandosz, T.J. Carbon textiles modified with copper-based reactive adsorbents as efficient media for detoxification of chemical warfare agents. ACS Appl. Mater. Interfaces 2017, 9, 26965–26973. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Hu, Y.; Florent, M.; Bandosz, T.J. Smart textiles of MOF/gC 3 N 4 nanospheres for the rapid detection/detoxification of chemical warfare agents. Nanoscale Horiz. 2017, 2, 356–364. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, Z.; Zhao, L.; Huang, M.; Dai, L.; Tian, D.; Zou, J.; Zeng, Y.; Hu, J.; Shen, F. Tailoring biochar by PHP towards the oxygenated functional groups (OFGs)-rich surface to improve adsorption performance. Chin. Chem. Lett. 2022, 33, 3097–3100. [Google Scholar] [CrossRef]
- Liatsou, I.; Pashalidis, I.; Oezaslan, M.; Dosche, C. Surface Characterization of Oxidized Biochar Fibers Derived from Luffa Cylindrica and Lanthanide Binding. J. Environ. Chem. Eng. 2017, 5, 4069–4074. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Kostoglou, N.; Thomas, A.; Rebholz, C.; Matthews, A.; Lewis, D.J. Flexible Nanoporous Activated Carbon for Adsorption of Organics from Industrial Effluents. Nanoscale 2021, 13, 15311–15323. [Google Scholar] [CrossRef]
- Hadjittofi, L.; Pashalidis, I. Uranium Sorption from Aqueous Solutions by Activated Biochar Fibres Investigated by FTIR Spectroscopy and Batch Experiments. J. Radioanal. Nucl. Chem. 2015, 304, 897–904. [Google Scholar] [CrossRef]
- ISO 9277:2022—Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method. International Standard Organization. Available online: https://www.iso.org/standard/71014.html (accessed on 23 November 2023).
- Kiliari, T.; Pashalidis, I. Simplified Alpha-Spectroscopic Analysis of Uranium in Natural Waters after Its Separation by Cation-Exchange. Radiat. Meas. 2010, 45, 966–968. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Ioannidis, I.; Pashalidis, I.; Raptopoulos, G.; Paraskevopoulou, P. Radioactivity/Radionuclide (U-232 and Am-241) Removal from Waters by Polyurea-Crosslinked Alginate Aerogels in the Sub-Picomolar Concentration Range. Gels 2023, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Kazakis, N.; Busico, G.; Ntona, M.M.; Philippou, K.; Kaprara, E.; Mitrakas, M.; Voudouris, K. The Origin of Uranium in Groundwater of the Eastern Halkidiki Region, Northern Greece. Sci. Total Environ. 2022, 812, 152445. [Google Scholar] [CrossRef]
- Ioannidis, I.; Anastopoulos, I.; Pashalidis, I. Microplastics as Radionuclide (U-232) Carriers. J. Mol. Liq. 2022, 351, 118641. [Google Scholar] [CrossRef]
- Ioannidis, I.; Xenofontos, A.; Anastopoulos, I.; Pashalidis, I. Americium Sorption by Microplastics in Aqueous Solutions. Coatings 2022, 12, 1452. [Google Scholar] [CrossRef]











| Material | SBET [m2/g] | VGurvich [nm] | W [nm] |
|---|---|---|---|
| Woven | 1119 | 0.47 | 0.84 |
| Ox-woven | 560 | 0.29 | 1.03 |
| Non-woven | 1845 | 0.86 | 0.93 |
| Ox-non-woven | 532 | 0.32 | 1.20 |
| Felt | 1080 | 0.49 | 0.90 |
| Ox-felt | 475 | 0.25 | 1.05 |
| Material | Element (at.%) | |||||
|---|---|---|---|---|---|---|
| C | N | Na | O | P | Si | |
| Woven | 83.5 | 3.6 | 0.1 | 11.7 | 0.9 | 0.2 |
| Ox-woven | 72.3 | 3.8 | -- | 23.8 | 0.2 | -- |
| Non-woven | 90.5 | 0.5 | 0.5 | 7.5 | 0.5 | 0.6 |
| Ox-non-woven | 75.6 | 1.6 | 0.1 | 22.6 | -- | -- |
| Felt | 89.6 | 1.3 | 0.3 | 8 | 0.5 | 0.2 |
| Ox-felt | 76.8 | 2.1 | -- | 21.1 | -- | -- |
| Material/pH | U(VI) | Am(III) | ||
|---|---|---|---|---|
| ΔH° (kJ/moL) | ΔS° (J/K·moL) | ΔH° (kJ/moL) | ΔS° (J/K·moL) | |
| Ox-woven/4 | 29 | 151 | 21 | 108 |
| Ox-non-woven/4 | 34 | 159 | 17 | 94 |
| Ox-felt/4 | 43 | 182 | 22 | 106 |
| Ox-woven/7 | 29 | 154 | 23 | 118 |
| Ox-non-woven/7 | 42 | 192 | 29 | 135 |
| Ox-felt/7 | 58 | 246 | 18 | 103 |
| Ox-woven/9 | 22 | 126 | 29 | 131 |
| Ox-non-woven/9 | 27 | 132 | 19 | 103 |
| Ox-felt/9 | 54 | 228 | 34 | 147 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidis, I.; Pashalidis, I.; Mulla, B.; Kotanidis, G.; Ioannou, K.; Constantinides, G.; Kostoglou, N.; Rebholz, C. Radionuclide Removal from Aqueous Solutions Using Oxidized Carbon Fabrics. Materials 2023, 16, 7479. https://doi.org/10.3390/ma16237479
Ioannidis I, Pashalidis I, Mulla B, Kotanidis G, Ioannou K, Constantinides G, Kostoglou N, Rebholz C. Radionuclide Removal from Aqueous Solutions Using Oxidized Carbon Fabrics. Materials. 2023; 16(23):7479. https://doi.org/10.3390/ma16237479
Chicago/Turabian StyleIoannidis, Ioannis, Ioannis Pashalidis, Batuhan Mulla, Gkerman Kotanidis, Kyriacos Ioannou, Georgios Constantinides, Nikolaos Kostoglou, and Claus Rebholz. 2023. "Radionuclide Removal from Aqueous Solutions Using Oxidized Carbon Fabrics" Materials 16, no. 23: 7479. https://doi.org/10.3390/ma16237479
APA StyleIoannidis, I., Pashalidis, I., Mulla, B., Kotanidis, G., Ioannou, K., Constantinides, G., Kostoglou, N., & Rebholz, C. (2023). Radionuclide Removal from Aqueous Solutions Using Oxidized Carbon Fabrics. Materials, 16(23), 7479. https://doi.org/10.3390/ma16237479