Synthesis of Poly-γ-Glutamic Acid and Its Application in Biomedical Materials
Abstract
:1. Introduction
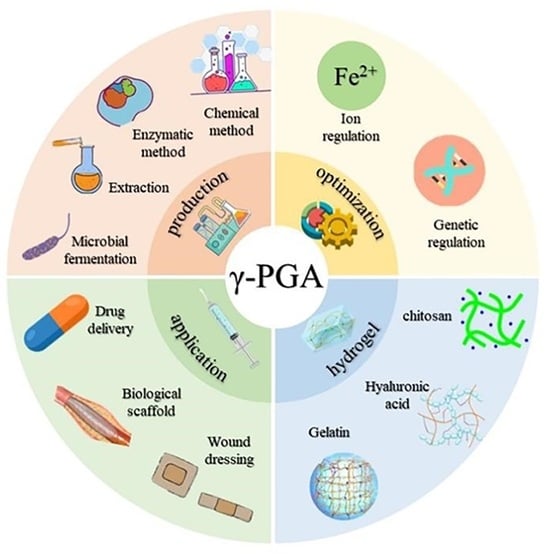
2. Preparation Methods of γ-PGA
2.1. Chemical Synthesis
2.2. Enzyme Conversion
2.3. Extraction
2.4. Microbial Fermentation
3. Optimize the Production of γ-PGA
3.1. Metabolic Process Regulation
3.2. Transforming Bacterial Strains

4. The γ-PGA Hydrogel
4.1. Crosslinking with Chitosan
4.2. Crosslinking with Hyaluronic Acid

4.3. Crosslinking with Gelatin
4.4. Crosslinking with Other Materials

5. PGA-Based Drug Delivery Systems
5.1. PGA-Based Anti-Tumor Drug Delivery


5.2. Delivery of Other Classes of Drugs Based on PGA

6. Tissue Engineering with PGA
6.1. Cartilage Tissue Engineering
6.2. Neural Tissue Engineering
6.3. Bone Tissue Engineering
7. PGA for Wound Healing Applications
7.1. Antibacterial Effect of PGA in Wound Healing

7.2. Other Roles of PGA in Wound Healing
8. Future Directions and Challenges
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tanaka, T.; Taniguchi, M. Poly(γ-glutamic acid) from Bacillus subtilis as an optically heterogeneous peptide in which D- and L-glutamic acid isomers are copolymerized into a single chain. Hydrocolloids 2000, 33138, 459–463. [Google Scholar]
- Ivanovics, G.; Erdos, L. Ein Beitrag zum Wesen der Kaspelsubstanz des Milzbrandbazillus. Z. Immuntatsforsch. 1937, 90, 5–19. [Google Scholar]
- Ho, G.-H.; Ho, T.-I.; Hsieh, K.-H.; Su, Y.-C.; Lin, P.-Y.; Yang, J.; Yang, K.-H.; Yang, S.-C. γ-Polyglutamic acid produced by Bacillus subtilis (natto): Structural characteristics, chemical properties and biological functionalities. J. Chin. Chem. Soc. 2006, 53, 1363–1384. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.B.; Fu, J.M.; Xie, J.C.; Ju, J.S.; Yu, B.; Wang, L.M. Efficient molasses utilization for low-molecular-weight poly-γ-glutamic acid production using a novel Bacillus subtilis stain. Microb. Cell Factories 2022, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Choi, Y.-H.; Shin, H.-J.; Poo, H.; Song, J.J.; Kim, C.-J.; Sung, M.-H. Effect of high-molecular-weight poly-γ-glutamic acid from Bacillus subtilis (chungkookjang) on Ca solubility and intestinal absorption. J. Microbiol. Biotechnol. 2005, 15, 855–858. [Google Scholar]
- Jin, H.D.; Ho, N.C.; Sang, Y.L. Efficient recovery of γ-poly (glutamic acid) from highly viscous culture broth. Biotechnol. Bioeng. 2001, 76, 219–223. [Google Scholar]
- Subramanian, G.; Hjelm, R.P.; Deming, T.J.; Gregory, S.S.; Li, Y.; Safinya, C. Structure of Complexes of Cationic Lipids and Poly(Glutamic Acid) Polypeptides: A Pinched Lamellar Phase. J. Am. Chem. Soc. 2000, 122, 26–34. [Google Scholar] [CrossRef]
- Xu, T.; Peng, F.; Zhang, T.; Chi, B.; Xu, H.; Mao, C.; Feng, S. Poly(γ-glutamic acid), coagulation? Anticoagulation? J. Biomater. Sci. Polym. Ed. 2016, 27, 1599–1610. [Google Scholar] [CrossRef]
- Honda, N.; Kawai, T.; Higashi, F. Preparation and polymerization of 7-membered cyclic carbamic carboxylic anhydride (gamma-nca) of glutamic-acid. Makromol. Chem. 1978, 179, 1643–1646. [Google Scholar] [CrossRef]
- Sanda, F.; Fujiyama, T.; Endo, T. Chemical synthesis of poly-gammaglutamic acid by polycondensation of gamma-glutamic acid dimer: Synthesis and reaction of poly-gamma-glutamic acid methyl ester. J. Polym. Sci. Polym. Chem. 2001, 39, 732–741. [Google Scholar] [CrossRef]
- Mao, Y.; Deng, M.C.; Zhang, Y.P. Poly-γ-glutamic Acid Biosynthesis by a Novel γ-glutamyltranspeptidase from Bacillus cereus. Biotechnology 2013, 12, 209–212. [Google Scholar]
- Kino, K.; Arai, T.; Arimura, Y. Poly-alpha-glutamic acid synthesis using a novel catalytic activity of Rim K from Escherichia coli K-12. Appl. Environ. Microbiol. 2011, 77, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yan, Q.J.; Wang, Y.C.; Li, Y.X.; Jiang, Z.Q. Efficient production of poly-γ-glutamic acid by Bacillus velezensis via solid-state fermentation and its application. Food Biosci. 2022, 46, 101575. [Google Scholar] [CrossRef]
- Moraes, L.P.; Alegre, R.M.; Brito, P.N. Optimisation of Poly(γ-Glutamic Acid) Production by Bacillus velezensis NRRL B-23189 in Liquid Fermentation with Molasses as the Carbon Source without Addition of Glutamic Acid. Int. Rev. Chem. Eng. Rapid Commun. 2012, 4, 618–623. [Google Scholar]
- Li, P.P.; Jiang, Y.; Wu, C.; Zhang, W.X.; Han, Y.L.; Wang, Q. Breeding of a γ-PGA-Producing strain and the effect of its fermentation on sandy soil moisture retention. J. Comput. Theor. Nanosci. 2016, 13, 5305–5311. [Google Scholar] [CrossRef]
- Ju, W.T.; Song, Y.S.; Jung, W.J.; Park, R.D. Enhanced production of poly-γ-glutamic acid by a newly-isolated Bacillus subtilis. Biotechnol. Lett. 2014, 36, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Xu, Z.; Cen, P. Efficient production of poly-gamma-glutamic acid by Bacillus subtilis ZJU-7. Appl. Biochem. Biotechnol. 2006, 133, 271–282. [Google Scholar] [CrossRef]
- Wang, D.; Fu, X.; Zhou, D.; Gao, J.; Bai, W. Engineering of a newly isolated Bacillus tequilensis BL01 for poly-γ-glutamic acid production from citric acid. Microb. Cell Fact. 2022, 21, 276. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, G.; Guo, Y.; Zhang, B.; Dong, M.; Wu, Y.; Wang, J.; Che, Z.; Liang, Z. Production of poly-γ-glutamic acid by a thermotolerant glutamate-independent strain and comparative analysis of the glutamate dependent difference. AMB Express 2017, 7, 213. [Google Scholar] [CrossRef]
- Guo, L.; Lu, L.; Wang, H.; Zhang, X.; Wang, G.; Zhao, T.; Zheng, G.; Qiao, C. Effects of Fe2+ addition to sugarcane molasses on poly-γ-glutamic acid production in Bacillus licheniformis CGMCC NO. 23967. Microb. Cell Fact. 2023, 22, 37. [Google Scholar] [CrossRef]
- Yang, G.; Chen, J.; Qu, Y.B.; Lun, S.Y. Effects of metal ions on gamma-poly (glutamic acid) synthesis by Bacillus licheniformis. Sheng Wu Gong Cheng Xue Bao. 2001, 17, 706–709. [Google Scholar] [PubMed]
- Meng, Y.; Dong, G.; Zhang, C.; Ren, Y.; Qu, Y.; Chen, W. Calcium regulates glutamate dehydrogenase and poly-γ-glutamic acid synthesis in Bacillus natto. Biotechnol. Lett. 2016, 38, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.; Raza, W.; Yu, G.; Ran, W.; Shen, Q.; Yang, X. Optimization of the production of poly-γ-glutamic acid by Bacillus amyloliquefaciens C1 in solid-state fermentation using dairy manure compost and monosodium glutamate production residues as basic substrates. Bioresour. Technol. 2011, 102, 7548–7554. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Liu, N.; Ke, L.; Zhao, X.; Qi, G. Microbial synthesis of poly-γ-glutamic acid (γ-PGA) with fulvic acid powder, the waste from yeast molasses fermentation. Biotechnol. Biofuels. 2020, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Ashiuchi, M.; Soda, K.; Misono, H. A Poly-γ-glutamate Synthetic System of Bacillus subtilis IFO 3336: Gene Cloning and Biochemical Analysis of Poly-γ-glutamate Produced by Escherichia coli Clone Cells. Biochem. Biophys. Res. Commun. 1999, 263, 6–12. [Google Scholar] [CrossRef]
- Thomas, C.; Michèle, M.; Agnès, F. CapE, a 47-amino-acid peptide, is necessary for Bacillus anthracis polyglutamate capsule synthesis. J. Bacteriol. 2005, 187, 7765–7772. [Google Scholar]
- Cao, M.F.; Geng, W.T.; Liu, L.; Song, C.J.; Xie, H.; Guoa, W.B.; Jin, Y.H.; Wang, S.F. Glutamic acid independent production of poly-γ-glutamic acid by Bacillus amyloliquefaciens LL3 and cloning of pgsBCA genes. Bioresour. Technol. 2011, 102, 4251–4257. [Google Scholar] [CrossRef]
- Scoffone, V.; Dondi, D.; Biino, G.; Borghese, G.; Pasini, D.; Galizzi, A.; Calvio, C. Knockout of pgdS and ggt genes improves γ-PGA yield in B. subtilis. Biotechnol. Bioeng. 2013, 110, 2006–2012. [Google Scholar] [CrossRef]
- Yoshihiro, O.; Joji, K.; Takeru, D.; Masayuki, A. Knockout of pgdS and ggt gene changes poly-γ-glutamic acid production in Bacillus licheniformis RK14-46. J. Biotechnol. 2019, 304, 57–62. [Google Scholar]
- Li, B.; Cai, D.; Chen, S. Metabolic Engineering of Central Carbon Metabolism of Bacillus licheniformis for Enhanced Production of Poly-γ-glutamic Acid. Appl. Biochem. Biotechnol. 2021, 193, 3540–3552. [Google Scholar] [CrossRef]
- Zhu, Y.; Du, S.; Yan, Y.; Pan, F.; Wang, R.; Li, S.; Xu, H.; Luo, Z. Systematic engineering of Bacillus amyloliquefaciens for efficient production of poly-γ-glutamic acid from crude glycerol. Bioresour. Technol. 2022, 359, 127382. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Gu, Y.; Quan, Y.; Gao, W.; Dang, Y.; Cao, M.; Lu, X.; Wang, Y.; Song, C.; Wang, S. Construction of energy-conserving sucrose utilization pathways for improving poly-γ-glutamic acid production in Bacillus amyloliquefaciens. Microb. Cell Fact. 2017, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, G.; Hua, J.; Wu, M.; Guo, W.; Gong, J.; Zhang, J.; Qiao, C. Preparation of γ-PGA hydrogels and swelling behaviors in salt solutions with different ionic valence numbers. RSC Adv. 2017, 7, 11085–11093. [Google Scholar] [CrossRef]
- Hahk, S.K.; Se, H.P.; Young, G.L. Polyelectrolyte complex hydrogel composed of chitosan and poly(γ-glutamic acid) for biological application: Preparation, physical properties, and cytocompatibility. J. Appl. Polym. Sci. 2007, 103, 386–394. [Google Scholar]
- Hye, S.K.; Ji, S.L.; Moon, I.K. Poly-γ-Glutamic Acid/Chitosan Hydrogel Nanoparticles Entrapping Glucose Oxidase and Magnetic Nanoparticles for Glucose Biosensing. J. Nanosci. Nanotechnol. 2020, 20, 5333–5337. [Google Scholar]
- Wang, M.; Zhang, E.; Yu, C.; Liu, D.; Zhao, S.; Xu, M.; Zhao, X.; Yue, W.; Nie, G. Dendrobium officinale Enzyme Changing the Structure and Behaviors of Chitosan/γ-poly(glutamic acid) Hydrogel for Potential Skin Care. Polymers 2022, 14, 2070. [Google Scholar] [CrossRef] [PubMed]
- Anne, D.; Danielle, F.; Odile, D.; Alain, D. Chitosan–chondroitin sulfate and chitosan–hyaluronate polyelectrolyte complexes: Biological properties. Biomaterials 1998, 19, 1275–1285. [Google Scholar]
- Matthew, H.W.; Salley, S.O.; Peterson, W.D.; Klein, M.D. Complex Coacervate Microcapsules for Mammalian Cell Culture and Artificial Organ Development. Biotechnol. Prog. 1993, 9, 510–519. [Google Scholar] [CrossRef]
- Bian, S.; He, M.; Sui, J.; Cai, H.; Sun, Y.; Liang, J. The self-crosslinking smart hyaluronic acid hydrogels as injectable three-dimensional scaffolds for cells culture. Colloids Surf. B Biointerfaces 2016, 140, 392–402. [Google Scholar] [CrossRef]
- Li, L.; Wang, N.; Jin, X.; Deng, R.; Nie, S.; Sun, L. Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials 2014, 35, 3903–3917. [Google Scholar] [CrossRef]
- Ma, X.B.; Liu, S.; Tang, H.J.; Yang, R.; Chi, B.; Ye, Z.W. In situ photocrosslinked hyaluronic acid and poly (gamma-glutamic acid) hydrogels as injectable drug carriers for load-bearing tissue application. J. Biomater. Sci. Polym. Ed. 2018, 29, 2252–2266. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.D.; Charati, M.B.; Kim, I.L.; Burdick, J.A. Injectable Shear–Thinning Hydrogels Engineered with a Self–Assembling Dock–and–Lock Mechanism. Biomaterials 2012, 33, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.B.; Liu, X.; Wang, P.H.; Wang, X.X.; Yang, R.; Liu, S.; Ye, Z.W.; Chi, B. Covalently Adaptable Hydrogel Based on Hyaluronic Acid and Poly (γ-glutamic acid) for Potential Load-Bearing Tissue Engineering. ACS Appl. Bio Mater. 2020, 3, 4036–4043. [Google Scholar] [CrossRef] [PubMed]
- Hiroyuki, K.; Yuki, A.; Yuko, K.-K.; Ung-il, C.; Takamasa, S. “Nonswellable” Hydrogel Without Mechanical Hysteresis. Science 2014, 343, 873–875. [Google Scholar]
- Haraguchi, K.; Takehisa, T. Nanocomposite Hydrogels: A Unique Organic-Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De-swelling Properties. Rep. Kawamura Inst. Chem. Res. 2002, 2002, 61–64. [Google Scholar] [CrossRef]
- Yang, J.J.; Lin, Y.Y.; Chao, K.H.; Wang, J.L. Gelatin-Poly (γ-Glutamic Acid) Hydrogel as a Potential Adhesive for Repair of Intervertebral Disc Annulus Fibrosus: Evaluation of Cytocompatibility and Degradability. Spine 2021, 46, E243–E249. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Li, F.; Hung, K.C.; Hsu, S.H.; Wang, J.L. Intervertebral disc needle puncture injury can be repaired using a gelatin–poly (γ-glutamic acid) hydrogel: An in vitro bovine biomechanical validation. Eur. Spine J. 2018, 27, 2631–2638. [Google Scholar] [CrossRef]
- Dou, C.Y.; Li, Z.; Luo, Y.; Gong, J.X.; Li, Q.J.; Zhang, J.F.; Zhang, Q.S.; Qiao, C.S. Bio-based poly (γ-glutamic acid)-gelatin double-network hydrogel with high strength for wound healing. Int. J. Biol. Macromol. 2022, 202, 438–452. [Google Scholar] [CrossRef]
- Chen, C.S.; Zeng, F.; Xiao, X.; Wang, Z.; Li, X.L.; Tan, R.W.; Liu, W.Q.; Zhang, Y.S.; She, Z.D.; Li, S.J. Three-Dimensionally Printed Silk-Sericin-Based Hydrogel Scaffold: A Promising Visualized Dressing Material for Real-Time Monitoring of Wounds. ACS Appl. Mater. Interfaces 2018, 10, 33879–33890. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, H.; Yu, F.Q.; Pei, Y.; Luo, X.G. Superclear, Porous Cellulose Membranes with Chitosan-Coated Nanofibers for Visualized Cutaneous Wound Healing Dressing. ACS Appl. Mater. Interfaces 2020, 12, 24370–24379. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, A.; Shin, W.; Heo, M.B.; Noh, H.J.; Hong, K.S.; Cho, J.H.; Lim, Y.T. Photothermal-modulated drug delivery and magnetic relaxation based on collagen/poly (γ-glutamic acid) hydrogel. Int. J. Nanomed. 2017, 12, 2607–2620. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Kontani, Y.; Ohmae, H.; Kawashima, S. Behavior of alginate gel beads containing chitosan salt prepared with water-soluble vitamins. Eur. J. Pharm. Biopharm. 2002, 53, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Tankhiwale, R. Investigation of water uptake behavior and stability of calcium alginate/chitosan bi-polymeric beads: Part-1. React. Funct. Polym. 2006, 66, 645–658. [Google Scholar] [CrossRef]
- Chan, W.P.; Kung, F.C.; Kuo, Y.L.; Yang, M.C.; Lai, W.F.T. Alginate/Poly(γ-glutamic Acid) Base Biocompatible Gel for Bone Tissue Engineering. BioMed. Res. Int. 2015, 2015, 185841. [Google Scholar] [CrossRef]
- Peng, S.-F.; Tseng, M.T.; Ho, Y.-C.; Wei, M.-C.; Liao, Z.X.; Sung, H.-W. Mechanisms of cellular uptake and intracellular trafficking with chitosan/DNA/poly(γ-glutamic acid) complexes as a gene delivery vector. Biomaterials 2011, 32, 239–248. [Google Scholar] [CrossRef]
- Li, C.; Yu, D.-F.; Newman, R.A.; Cabral, F.S. Complete regression of well-established tumors using a novel water-soluble poly(L-glutamic acid)–paclitaxel conjugate. Cancer Res. 1998, 58, 2404–2409. [Google Scholar]
- Singer, J.W. Paclitaxel poliglumex (XYOTAX, CT-2103): A macromolecular taxane. J. Control Release 2005, 109, 120–126. [Google Scholar] [CrossRef]
- Arroyo-Crespo, J.J.; Deladriere, C.; Nebot, V.J.; Charbonnier, D.; Masiá, E.; Paul, A. Anticancer Activity Driven by Drug Linker Modification in a Polyglutamic Acid-Based Combination-Drug Conjugate. Adv. Funct. Mater. 2018, 28, 1800931. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Shafaroodi, H.; Asadi, S.; Nezami, B.G.; Ghasemi, M.; Rahimpour, S.; Dehpour, A.R. Sildenafil decreased cardiac cell apoptosis in diabetic mice: Reduction of oxidative stress as a possible mechanism. Can. J. Physiol. Pharmacol. 2009, 87, 556–564. [Google Scholar] [CrossRef]
- Nisar, S. Gamma-Radiation induced L-glutamic acid grafted highly porous, pH-responsive chitosan hydrogel beads: A smart and biocompatible vehicle for controlled anti-cancer drug delivery. Int. J. Biol. Macromol. 2021, 182, 37–50. [Google Scholar] [CrossRef]
- Guo, Y. Hydrophilic Poly(glutamic acid)-Based Nanodrug Delivery System: Structural Influence and Antitumor Efficacy. Polymers 2022, 14, 2242. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Liu, Y.; Tan, S.; Qu, R.; Wu, Z.; Zhou, Y.; Huang, J. Preparation of PGA-PAE-Micelles for Enhanced Antitumor Efficacy of Cisplatin. ACS Appl. Mater. Interfaces 2018, 10, 25006–25016. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Dual Stable Nanomedicines Prepared by Cisplatin-Crosslinked Camptothecin Prodrug Micelles for Effective Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 20649–20659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, X.; Wu, S.; Chen, Y.; Tan, S.; Liu, Y.; Huang, J. Fabrication and evaluation of a gamma-PGA-based self-assembly transferrin receptor-targeting anticancer drug carrier. Int. J. Nanomed. 2018, 13, 7873–7889. [Google Scholar] [CrossRef]
- Wang, R.; He, D.; Wang, H.; Wang, J.; Yu, Y.; Chen, Q.; Sun, C.; Shen, Y.; Tu, J.; Xiong, Y. Redox-sensitive polyglutamic acid-platinum (IV) prodrug grafted nanoconjugates for efficient delivery of cisplatin into breast tumor. Nanomedicine 2020, 29, 102252. [Google Scholar] [CrossRef]
- Jiang, Z.; Feng, X.; Zou, H.; Xu, W.; Zhuang, X. Poly(l-glutamic acid)-cisplatin nanoformulations with detachable PEGylation for prolonged circulation half-life and enhanced cell internalization. Bioact. Mater. 2021, 6, 2688–2697. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Sun, J.; Zheng, M.; Cui, L.; Zhang, Y.; Chen, X. Organic-solvent-free “Lego-like” Modular Preparation of Fab-nondestructive Antibody-Drug Conjugates with Ultra-High Drug-To-Antibody Ratio. Adv. Mater. 2023, 2023, e2300377. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, B.; Niu, L.; Tang, C.; McMullen, P.; Jain, P.; Jiang, S. Zwitterionic Peptide Cloak Mimics Protein Surfaces for Protein Protection. Angew. Chem. Int. Ed. Engl. 2020, 59, 22378–22381. [Google Scholar] [CrossRef]
- Tyrel, T.; Stephan, S.B.; Moffett, H.F.; McKnight, L.E.; Ji, W.; Reiman, D.; Stephan, M.T. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 2017, 12, 813–820. [Google Scholar]
- Nieto-Orellana, A.; Li, H.; Rosiere, R.; Wauthoz, N.; Williams, H.; Monteiro, C.J.; Stolnik, S. Targeted PEG-poly(glutamic acid) complexes for inhalation protein delivery to the lung. J. Control. Release 2019, 316, 250–262. [Google Scholar] [CrossRef]
- Enshaei, H.; Molina, B.G.; Puiggalí-Jou, A.; Saperas, N.; Aleman, C. Polypeptide hydrogel loaded with conducting polymer nanoparticles as electroresponsive delivery system of small hydrophobic drugs. Eur. Polym. J. 2022, 173, 111199. [Google Scholar] [CrossRef]
- Das, M.P.; Pandey, G.; Neppolian, B.; Das, J. Design of poly-l-glutamic acid embedded. mesoporous bioactive glass nanospheres for pH-stimulated chemotherapeutic drug delivery and antibacterial susceptibility. Colloids Surf B Biointerfaces 2021, 202, 111700. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yan, X.; Zhao, J.; Feng, H.; Li, P.; Tong, Z.; Jin, S. Preparation of the chitosan/poly(glutamic acid)/alginate polyelectrolyte complexing hydrogel and study on its drug releasing property. Carbohydr. Polym. 2018, 191, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Guo, K.; Zhang, W.; He, Y.; Yang, K.; Chen, Q.; Xu, H. Poly-γ-Glutamic Acid Microgel-Encapsulated Probiotics with Gastric Acid Resistance and Smart Inflammatory Factor Targeted Delivery Performance to Ameliorate Colitis. Adv. Funct. Mater. 2022, 32, 2113034. [Google Scholar] [CrossRef]
- Ávila, H.M.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 2016, 1, 22–35. [Google Scholar] [CrossRef]
- Yang, R.; Wang, X.; Liu, S.; Zhang, W.; Wang, P.; Liu, X.; Ren, Y.; Tan, X.; Chi, B. Bioinspired poly (γ-glutamic acid) hydrogels for enhanced chondrogenesis of bone marrow-derived mesenchymal stem cells. Int. J. Biol. Macromol. 2020, 142, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Saeed, A.O.; Hassan, W.; Keigher, C.; Zheng, Y.; Tai, H.; Pandit, A.; Wang, W. “one-step” preparation of thiolene clickable PEG-based thermoresponsive hyperbranched copolymer for in situ crosslinking hybrid hydrogel. Eur. Polym. Fed. 2012, 33, 120–126. [Google Scholar]
- Lee, A.K.; Lin, Y.H.; Tsai, C.H.; Chang, W.T.; Lin, T.L.; Shie, M.Y. Digital Light Processing Bioprinted Human Chondrocyte-Laden Poly (γ-Glutamic Acid)/Hyaluronic Acid Bio-Ink towards Cartilage Tissue Engineering. Biomedicines 2021, 9, 714. [Google Scholar] [CrossRef]
- Ma, X.B.; Yang, R.; Sekhar, K.P.; Chi, B. Injectable Hyaluronic Acid/Poly(γ-glutamic acid) Hydrogel with Step-by-step Tunable Properties for Soft Tissue Engineering. Chin. J. Polym. Sci. 2021, 39, 957–965. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Ku, H.F.; Rajesh, R. Chitosan/γ-poly(glutamic acid) scaffolds with surface-modified albumin, elastin and poly-l-lysine for cartilage tissue engineering. Mater. Sci. Eng. C 2017, 78, 265–277. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Chang, Y.H. Differentiation of induced pluripotent stem cells toward neurons in hydrogel biomaterials. Colloids Surf. B Biointerfaces 2013, 102, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Ma, T.L.; Tan, D.H.; Su, A.A.; Washington, K.M. Injectable Hydrogel Guides Neurons Growth with Specific Directionality. Int. J. Mol. Sci. 2023, 24, 7952. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Chang, Y.Y.; Wu, J.G.; Lin, C.Y.; An, H.L.; Luo, S.C.; Tang, T.K.; Su, W.F. Novel 3D Neuron Regeneration Scaffolds Based on Synthetic Polypeptide Containing Neuron Cue. Macromol. Biosci. 2018, 18, 1616–5195. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, J.; Gan, D. The characteristics of mussel-inspired nHA/OSA injectable hydrogel and repaired bone defect in rabbit. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1814–1825. [Google Scholar] [CrossRef] [PubMed]
- Bo, W.; Jia, L.; Dong, Y.; Wu, N.Q. Mussel-Inspired Bisphosphonated Injectable Nanocomposite Hydrogels with Adhesive, Self-Healing, and Osteogenic Properties for Bone Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 32673–32689. [Google Scholar]
- Bae, S.R.; Park, C.; Choi, J.C.; Poo, H.; Kim, C.J.; Sung, M.H. Effects of ultra high molecular weight poly-gamma-glutamic acid from Bacillus subtilis (chungkookjang) on corneal wound healing. J. Microbiol. Biotechnol. 2010, 20, 803–808. [Google Scholar] [PubMed]
- Shi, L.; Yang, N.; Zhang, H.; Chen, L.; Tao, L.; Wei, Y.; Liu, H.; Luo, Y. A novel poly(γ-glutamic acid)/silk-sericin hydrogel for wound dressing: Synthesis, characterization and biological evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 533–540. [Google Scholar] [CrossRef]
- Sun, A.; He, X.; Li, L.; Li, T.; Liu, Q.; Zhou, X.; Qian, Z. An injectable photopolymerized hydrogel with antimicrobial and biocompatible. properties for infected skin regeneration. NPG Asia Materials. 2020, 12, 1884–4049. [Google Scholar] [CrossRef]
- Sun, A.; Hu, D.; He, X.; Ji, X.; Li, T.; Wei, X.; Qian, Z. Mussel-inspired hydrogel with injectable self-healing and antibacterial properties promotes wound healing in burn wound infection. NPG Asia Mater. 2022, 14, 86. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; He, Y.; Wang, L.; Deng, Z.; Liu, J.; Cai, K. Polydopamine Nanosheets Doped Injectable Hydrogel with Nitric Oxide Release and Photothermal Effects for Bacterial Ablation and Wound Healing. Adv. Health Mater. 2021, 10, e2101476. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Z.; Chen, X.; Feng, K.; Hu, T.; Huang, B.; Tang, J.; Wang, G.; Liu, S.; Yang, G.; et al. Double-network cellulose-based hybrid hydrogels with favourable biocompatibility and antibacterial activity for wound healing. Carbohydr. Polym. 2023, 319, 121193. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Y.; Cornel, E.J.; Li, C.; Du, J. Combined Antioxidant-Antibiotic Treatment for Effectively Healing Infected Diabetic Wounds Based on Polymer Vesicles. ACS Nano. 2021, 15, 9027–9038. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Monroe, M.B.B. Bacterial protease-responsive shape memory polymers for infection surveillance and biofilm inhibition in chronic wounds. J. Biomed. Mater. Res. A 2023, 111, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Yang, J.; Lin, L.; Wang, R.; Cheng, B.; Chen, Y.; Ma, X. In situ synthesis of poly (gamma- glutamic acid)/alginate/AgNP composite microspheres with antibacterial and hemostatic properties. Carbohydr. Polym. 2019, 221, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wu, J.; Deng, M.; Wang, P.; Ji, G.; Wang, M.; Zhou, C.; Blum, N.T.; Zhang, W.; Shi, H.; et al. Multifunctional Magnesium Organic Framework-Based Microneedle Patch for Accelerating Diabetic Wound Healing. ACS Nano. 2021, 23, 17842–17853. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Chen, T.; Wang, Y.; Xu, Q.; Feng, B.; Weng, J.; Wang, J. By Endowing Polyglutamic Acid/Polylysine Composite Hydrogel with Super Intrinsic Characteristics to Enhance its Wound Repair Potential. Macromol. Biosci. 2021, 21, e2000367. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A composite hydrogel of chitosan/heparin/poly (gamma-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr. Polym. 2018, 180, 168–174. [Google Scholar] [CrossRef]
- Yang, R.; Huang, J.; Zhang, W.; Xue, W.; Jiang, Y.; Li, S.; Chi, B. Mechanoadaptive injectable hydrogel based on poly(gamma-glutamic acid) and hyaluronic acid regulates fibroblast migration for wound healing. Carbohydr. Polym. 2021, 273, 118607. [Google Scholar] [CrossRef]
- Yang, R.; Huang, J.; Zhang, W.; Xue, W.; Jiang, Y.; Li, S.; Chi, B. Injectable adaptive self-healing hyaluronic acid/poly (gamma-glutamic acid) hydrogel for cutaneous wound healing. Acta Biomater. 2021, 127, 102–115. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Zhang, X.; Tang, P.; Zheng, T.; Ran, R.; Li, G. An injectable, self-healing, and antioxidant collagen- and hyaluronic acid-based hydrogel mediated with gallic acid and dopamine for wound repair. Carbohydr. Polym. 2023, 320, 121231. [Google Scholar] [CrossRef]
- Xu, T.; Yang, R.; Ma, X.; Chen, W.; Liu, S.; Liu, X.; Chi, B. Bionic Poly(gamma-Glutamic Acid) Electrospun Fibrous Scaffolds for Preventing Hypertrophic Scars. Adv. Health Mater. 2019, 8, e1900123. [Google Scholar] [CrossRef] [PubMed]



| Synthesis Methods | Purity | Productive | Cost | Pollution | Applications |
|---|---|---|---|---|---|
| Chemical synthesis | Low | Low | High | High | Occasionally laboratory study |
| Enzyme conversion | High | High | High | Low | Soil, plant regulators, etc. |
| Extraction | Low | Low | Low | Low | Early production methods of γ-PGA |
| Microbial fermentation | High | Relatively high | Relatively low | Low | Commonly used for large-scale production of γ-PGA and applied in cosmetics, food, pharmaceuticals, water treatment, and other fields |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Han, Y.; Zheng, X.; Xue, B.; Zhang, X.; Mahmut, Z.; Wang, Y.; Dong, B.; Zhang, C.; Gao, D.; et al. Synthesis of Poly-γ-Glutamic Acid and Its Application in Biomedical Materials. Materials 2024, 17, 15. https://doi.org/10.3390/ma17010015
Cai M, Han Y, Zheng X, Xue B, Zhang X, Mahmut Z, Wang Y, Dong B, Zhang C, Gao D, et al. Synthesis of Poly-γ-Glutamic Acid and Its Application in Biomedical Materials. Materials. 2024; 17(1):15. https://doi.org/10.3390/ma17010015
Chicago/Turabian StyleCai, Minjian, Yumin Han, Xianhong Zheng, Baigong Xue, Xinyao Zhang, Zulpya Mahmut, Yuda Wang, Biao Dong, Chunmei Zhang, Donghui Gao, and et al. 2024. "Synthesis of Poly-γ-Glutamic Acid and Its Application in Biomedical Materials" Materials 17, no. 1: 15. https://doi.org/10.3390/ma17010015
APA StyleCai, M., Han, Y., Zheng, X., Xue, B., Zhang, X., Mahmut, Z., Wang, Y., Dong, B., Zhang, C., Gao, D., & Sun, J. (2024). Synthesis of Poly-γ-Glutamic Acid and Its Application in Biomedical Materials. Materials, 17(1), 15. https://doi.org/10.3390/ma17010015