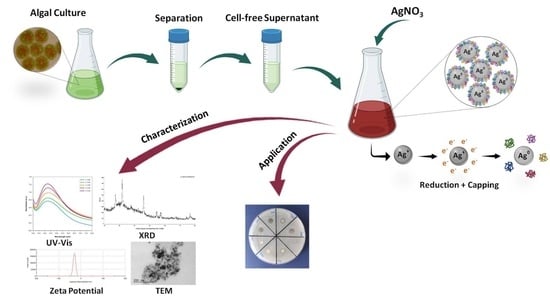
Green Synthesis of Silver Nanoparticles Using the Cell-Free Supernatant of Haematococcus pluvialis Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organism and Growth Conditions
2.2. Synthesis of AgNPs
2.3. Characterization of AgNPs
2.4. Long-Term Stability of the Biosynthesized AgNPs
2.5. Antibacterial Activity of AgNPs
3. Results and Discussion
3.1. Biosynthesis of AgNPs by the Cell-Free Supernatant of H. pluvialis Culture
3.1.1. Effect of Illumination Conditions
3.1.2. Effect of Temperature
3.1.3. Effect of pH
3.1.4. Effect of Metal Precursor Concentration
3.1.5. Effect of AgNO3 Aqueous Solution tο Cell-Free Supernatant Ratio
3.1.6. Effect of Stirring
3.1.7. Summary of Optimal Conditions for the Synthesis of AgNPs by the CFS of H. pluvialis Culture
3.2. Characterization of Biosynthesized AgNPs
3.2.1. Zeta Potential and Polydispersity Index (PDI) of the AgNPs
3.2.2. XRD Analysis
3.2.3. TEM Analysis
3.3. Long-Term Stability of the Biosynthesized AgNPs
3.4. Antibacterial Activity of the Biosynthesized AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taghizadeh, S.M.; Morowvat, M.H.; Negahdaripour, M.; Ebrahiminezhad, A.; Ghasemi, Y. Biosynthesis of metals and metal Oxide nanoparticles through microalgal nanobiotechnology: Quality control aspects. BioNanoScience 2021, 11, 209–226. [Google Scholar] [CrossRef]
- Gong, D.; Sun, L.; Li, X.; Zhang, W.; Zhang, D.; Cai, J. Micro/Nanofabrication, Assembly, and Actuation Based on Microorganisms: Recent Advances and Perspective. Small Struct. 2023, 4, 2200356. [Google Scholar] [CrossRef]
- Naveed, M.; Makhdoom, S.I.; Rehman, S.u.; Aziz, T.; Bashir, F.; Ali, U.; Alharbi, M.; Alshammari, A.; Alasmari, A.F. Biosynthesis and Mathematical Interpretation of Zero-Valent Iron NPs Using Nigella sativa Seed Tincture for Indemnification of Carcinogenic Metals Present in Industrial Effluents. Molecules 2023, 28, 3299. [Google Scholar] [CrossRef] [PubMed]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interfac. 2022, 300, 102597. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.J.P.; Pratibha, S.; Rawat, J.M.; Venugopal, D.; Sahu, P.; Gowda, A.; Qureshi, K.A.; Jaremko, M. Recent Advances in Green Synthesis, Characterization, and Applications of Bioactive Metallic Nanoparticles. Pharmaceuticals 2022, 15, 455. [Google Scholar] [CrossRef] [PubMed]
- Abuzeid, H.M.; Julien, C.M.; Zhu, L.; Hashem, A.M. Green Synthesis of Nanoparticles and Their Energy Storage, Environmental, and Biomedical Applications. Crystals 2023, 13, 1576. [Google Scholar] [CrossRef]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green Synthesis of Metallic Nanoparticles: Applications and Limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Vishwanath, R.; Negi, B. Conventional and green methods of synthesis of silver nanoparticles and their antimicrobial properties. Curr. Res. Green Sustain. Chem. 2021, 4, 100205. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Garg, D.; Sarkar, A.; Chand, P.; Bansal, P.; Gola, D.; Sharma, S.; Khantwal, S.; Surabhi; Mehrotra, R.; Chauhan, N.; et al. Synthesis of silver nanoparticles utilizing various biological systems: Mechanisms and applications—A review. Prog. Biomater. 2020, 9, 81–95. [Google Scholar] [CrossRef]
- Husain, S.; Nandi, A.; Simnani, F.Z.; Saha, U.; Ghosh, A.; Sinha, A.; Sahay, A.; Samal, S.K.; Panda, P.K.; Verma, S.K. Emerging Trends in Advanced Translational Applications of Silver Nanoparticles: A Progressing Dawn of Nanotechnology. J. Funct. Biomater. 2023, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Upadhyay, A.; Hussain, A.; Bag, S.; Chaterjee, D.; Rihan, M.; Mishra, N.; Bhatt, S.; Puri, V.; Sharma, A.; et al. Green biogenic silver nanoparticles, therapeutic uses, recent advances, risk assessment, challenges, and future perspectives. J. Drug Deliv. Sci. Tec. 2022, 77, 103876. [Google Scholar] [CrossRef]
- Vidyasagar; Patel, R.R.; Singh, S.K.; Singh, M. Green synthesis of silver nanoparticles: Methods, biological applications, delivery and toxicity. Mater. Adv. 2023, 4, 1831–1849. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Fais, G.; Casula, M.; Borselli, M.; Giannaccare, G.; Locci, A.M.; Lai, N.; Orrù, R.; Cao, G.; Concas, A. Nanoparticles from microalgae and their biomedical applications. Mar. Drugs 2023, 21, 352. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Lan, C.Q. Advances in biosynthesis of noble metal nanoparticles mediated by photosynthetic organisms-A review. Colloid Surf. B 2019, 184, 110519. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Rai, L.C.; Dubey, S.K. Cyanobacteria: Miniature factories for green synthesis of metallic nanomaterials: A review. Biometals 2022, 35, 653–674. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for high-value products: A way towards green nutraceutical and pharmaceutical compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Morais, W.G., Jr.; Martins, A.A.; Caetano, N.S.; Mata, T.M. Microalgae biomolecules: Extraction, separation and purification methods. Processes 2021, 9, 10. [Google Scholar] [CrossRef]
- Hachicha, R.; Elleuch, F.; Ben Hlima, H.; Dubessay, P.; de Baynast, H.; Delattre, C.; Pierre, G.; Hachicha, R.; Abdelkafi, S.; Michaud, P.; et al. Biomolecules from microalgae and cyanobacteria: Applications and market survey. Appl. Sci. 2022, 12, 1924. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, D.; Sasmal, S. A Review of green synthesis of metal nanoparticles using algae. Front. Microbiol. 2021, 12, 693899. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, E.; Priyadarshini, S.S.; Pradhan, N. Heavy metal resistance in algae and its application for metal nanoparticle synthesis. Appl. Microbiol. Biotechnol. 2019, 103, 3297–3316. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Shahid, A.; Zhu, H.; Wang, N.; Javed, M.R.; Ahmad, N.; Xu, N.; Alam, M.A.; Mehmood, M.A. Prospects of algae-based green synthesis of nanoparticles for environmental applications. Chemosphere 2022, 293, 133571. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Afreen, S.; Yasin, D.; Afzal, B.; Fatma, T. Cyanobacteria as a bioreactor for synthesis of silver nanoparticles-an effect of different reaction conditions on the size of nanoparticles and their dye decolorization ability. J. Microbiol. Methods 2019, 162, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Oslan, S.N.H.; Shoparwe, N.F.; Yusoff, A.H.; Rahim, A.A.; Chang, C.S.; Tan, J.S.; Oslan, S.N.; Arumugam, K.; Ariff, A.B.; Sulaiman, A.Z.; et al. A Review on Haematococcus pluvialis bioprocess optimization of green and red stage culture conditions for the production of natural astaxanthin. Biomolecules 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Deng, J.; Huang, J.; Wu, Z.; Yi, L.; Bi, Y.; Chen, F. Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: Advances and outlook. Bioresour. Technol. 2021, 340, 125736. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef]
- Cheng, W.H.; Wong, L.S.; Lau, M.T.; Chong, M.Y.; Ong, G.H. Effects of silver nanoparticles on the carotenoid production from Haematococcus pluvialis. EnvironmentAsia 2020, 13, 106–111. [Google Scholar] [CrossRef]
- Venckus, P.; Endriukaitytė, I.; Čekuolytė, K.; Gudiukaitė, R.; Pakalniškis, A.; Lastauskienė, E. Effect of biosynthesized silver nanoparticles on the growth of the green microalga Haematococcus pluvialis and astaxanthin synthesis. Nanomaterials 2023, 13, 1618. [Google Scholar] [CrossRef]
- Savvidou, M.G.; Ferraro, A.; Hristoforou, E.; Mamma, D.; Kekos, D.; Kolisis, F.N. Incorporation of magnetic nanoparticles into protoplasts of microalgae Haematococcus pluvialis: A tool for biotechnological applications. Molecules 2020, 25, 5068. [Google Scholar] [CrossRef] [PubMed]
- Mora-Godínez, S.; Abril-Martínez, F.; Pacheco, A. Green synthesis of silver nanoparticles using microalgae acclimated to high CO2. Mater. Today Proc. 2022, 48, 5–9. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F.; Moawad, H.; Oh, Y.-K. Influence of nitrogen source and growth phase on extracellular biosynthesis of silver nanoparticles using cultural filtrates of Scenedesmus obliquus. Appl. Sci. 2019, 9, 1465. [Google Scholar] [CrossRef]
- Rajkumar, R.; Ezhumalai, G.; Gnanadesigan, M. A green approach for the synthesis of silver nanoparticles by Chlorella vulgaris and its application in photocatalytic dye degradation activity. Environ. Technol. Innov. 2021, 21, 101282. [Google Scholar] [CrossRef]
- Patel, V.; Berthold, D.; Puranik, P.; Gantar, M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2015, 5, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Navarro Gallón, S.M.; Alpaslan, E.; Wang, M.; Larese-Casanova, P.; Londoño, M.E.; Atehortúa, L.; Pavóne, J.J.; Webster, T.J. Characterization and study of the antibacterial mechanisms of silver nanoparticles prepared with microalgal exopolysaccharides. Mater. Sci. Eng. C 2019, 99, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.C.; Figueiredo, R.C.; Ribeiro-Andrade, R.; Pontes-Silva, A.V.; Arantes, M.L.; Giani, A.; Figueredo, C.C. High diversity of microalgae as a tool for the synthesis of different silver nanoparticles: A species-specific green synthesis. Colloids Interface Sci. Commun. 2021, 42, 100420. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Almohawes, Z.N.; Alahdal, H.; Momenah, M.A.; Bin-Meferij, M.M. Green Synthesis of hexagonal silver nanoparticles using a novel microalgae Coelastrella aeroterrestrica strain BA_Chlo4 and resulting anticancer, antibacterial, and antioxidant activities. Pharmaceutics 2022, 14, 2002. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Lin, J.; Dahoumane, S.A.; Jeffryes, C. A mechanistic view of the light-induced synthesis of silver nanoparticles using extracellular polymeric substances of Chlamydomonas reinhardtii. Molecules 2019, 24, 3506. [Google Scholar] [CrossRef]
- Shantkriti, S.; Pradeep, M.; Unish, K.K.; Viji Das, M.S.; Nidhin, S.; Gugan, K.; Murugan, A. Biosynthesis of silver nanoparticles using Dunaliella salina and its antibacterial applications. Appl. Surf. Sci. Adv. 2023, 13, 100377. [Google Scholar] [CrossRef]
- Husain, S.; Sardar, M.; Fatma, T. Screening of cyanobacterial extracts for synthesis of silver nanoparticles. World J. Microbiol. Biotechnol. 2015, 31, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Tiwari, R.; Kumar, V.; Singh, P.; Riyazat Khadim, S.K.; Tiwari, A.; Srivastava, V.; Hasan, S.H.; Asthana, R.K. Photo-induced biosynthesis of silver nanoparticles from aqueous extract of Dunaliella salina and their anticancer potential. J. Photochem. Photobiol. B Biol. 2017, 166, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Lan, C.Q. Mechanism of light-dependent biosynthesis of silver nanoparticles mediated by cell extract of Neochloris oleoabundans. Colloids Surf. B 2018, 170, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Cao, J.; Kang, G.; Lan, C.Q. Effects of reaction conditions on light-dependent silver nanoparticle biosynthesis mediated by cell extract of green alga Neochloris oleoabundans. Environ. Sci. Pollut. Res. 2019, 26, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, Z. Silver nanoparticles to self-assembled films: Green synthesis and characterization. Colloids Surf. B 2012, 90, 48. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, S.A.; Kotakadi, V.S.; Subramanyam, G.K.; Penchalaneni, J.; Challagundla, V.N.; Dvr, S.G.; Pasupuleti, V.R. Multifaceted phytogenic silver nanoparticles by an insectivorous plant Drosera spatulata Labill var. bakoensis and its potential therapeutic applications. Sci. Rep. 2021, 11, 21969. [Google Scholar] [CrossRef] [PubMed]
- Velgosova, O.; Dolinská, S.; Mražíková, A.; Briančin, J. Effect of P. kessleri extracts treatment on AgNPs synthesis. Inorg. Nano-Met. Chem. 2020, 50, 842–852. [Google Scholar] [CrossRef]
- Prasad, T.N.; Kambala, V.S.R.; Naidu, R. Phyconanotechnology: Synthesis of silver nanoparticles using brown marine algae Cystophora moniliformis and their characterisation. J. Appl. Phycol. 2013, 25, 177–182. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; Abo-elmagd, R.A.; Bawazir, R.R. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.-W.; Yu, H.-Q.; Sheng, G.-P. Light-induced reduction of silver ions to silver nanoparticles in aquatic environments by microbial extracellular polymeric substances (EPS). Water Res. 2016, 106, 242–248. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Mills, G.; Hajek, B. Spontaneous formation of silver particles in basic 2-propanol. J. Phys. Chem. 1993, 97, 11542–11550. [Google Scholar] [CrossRef]
- Aboelfetoh, E.E.; El-Shenody, R.A.; Ghobara, M.M. Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): Reaction optimization, catalytic and antibacterial activities. Environ. Monit. Assess. 2017, 189, 349. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Bagheri, M.; Taghizadeh, S.-M.; Berenjian, A.; Ghasemi, Y. Biomimetic synthesis of silver nanoparticles using microalgal secretory carbohydrates as a novel anticancer and antimicrobial. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 015018. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.A.; Jeffryes, C. Individual and combined effects of extracellular polymeric substances and whole cell components of Chlamydomonas reinhardtii on silver nanoparticle synthesis and stability. Molecules 2019, 24, 956. [Google Scholar] [CrossRef]
- Lotfy, W.A.; Alkersh, B.M.; Sabry, S.A.; Ghozlan, H.A. Biosynthesis of silver nanoparticles by Aspergillus terreus: Characterization, optimization, and biological activities. Front. Bioeng. Biotechnol. 2021, 9, 633468. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Tiwari, R.; Singh, V.K.; Singh, P.; Khadim, S.R.; Singh, U.; Laxmi; Srivastava, V.; Hasan, S.H.; Asthana, R.K. Green synthesis of gold nanoparticles from Dunaliella salina, its characterization and in vitro anticancer activity on breast cancer cell line. Drug Deliv. Sci. Technol. 2019, 51, 164–176. [Google Scholar] [CrossRef]
- Gemishev, O.; Panayotova, M.; Gicheva, G.; Mintcheva, N. Green Synthesis of stable spherical monodisperse silver nanoparticles using a cell-free extract of Trichoderma reesei. Materials 2022, 15, 481. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kaner, R.B. Shape and aggregation control of nanoparticles: Not shaken, not Stirred. J. Am. Chem. Soc. 2006, 128, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Don, M.M. Optimization of process variables for the synthesis of silver nanoparticles by Pycnoporus sanguineus using statistical experimental design. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 11–20. [Google Scholar] [CrossRef]
- EL-Moslamy, S.; Elkady, M.; Rezk, A.; Abdel-Fattah, Y.R. Applying Taguchi design and large-scale strategy for mycosynthesis of nano-silver from endophytic Trichoderma harzianum SYA.F4 and its application against phytopathogens. Sci. Rep. 2017, 7, 45297. [Google Scholar] [CrossRef] [PubMed]
- Urnukhsaikhan, E.; Bold, B.E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, S. Green fabrication of bioactive silver nanoparticles using Mentha pulegium extract under alkaline: An enhanced anticancer activity. ACS Omega 2022, 7, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee on Powder Diffraction Standards, JCPDS file. No. 04-0783.
- Khalid, M.; Khalid, N.; Ahmed, I.; Hanif, A.; Ismail, M.; Janjua, H.A. Comparative studies of three novel freshwater microalgae strains for synthesis of silver nanoparticles: Insights of characterization, antibacterial, cytotoxicity and antiviral activities. J. Appl. Phycol. 2017, 29, 1851–1863. [Google Scholar] [CrossRef]
- Muthusamy, G.; Thangasamy, S.; Raja, M.; Chinnappan, S.; Kandasamy, S. Biosynthesis of silver nanoparticles from Spirulina microalgae and its antibacterial activity. Environ. Sci. Pollut. Res. 2017, 24, 19459–19464. [Google Scholar] [CrossRef] [PubMed]
- Habibullah, G.; Viktorova, J.; Ulbrich, P.; Ruml, T. Effect of the physicochemical changes in the antimicrobial durability of green synthesized silver nanoparticles during their long-term storage. RSC Adv. 2022, 12, 30386–30403. [Google Scholar] [CrossRef] [PubMed]
- Izak-Nau, E.; Huk, A.; Reidy, B.; Uggerud, H.; Vadset, M.; Eiden, S.; Voetz, M.; Himly, M.; Duschl, A.; Dusinska, M.; et al. Impact of storage conditions and storage time on silver nanoparticles’ physicochemical properties and implications for their biological effects. RSC Adv. 2015, 5, 84172–84185. [Google Scholar] [CrossRef]
- Velgosova, O.; Mražíková, A.; Čižmárová, E.; Málek, J. Green synthesis of Ag nanoparticles: Effect of algae life cycle on Ag nanoparticle production and long-term stability. Trans. Nonferrous Met. Soc. China 2018, 28, 974–979. [Google Scholar] [CrossRef]
- Tripathi, N.; Goshisht, M.K. Recent advances and mechanistic insights into antibacterial activity, antibiofilm activity, and cytotoxicity of silver nanoparticles. ACS Appl. Bio Mater. 2022, 5, 1391–1463. [Google Scholar] [CrossRef]













| Species | Synthesis Conditions | Shape | Size (nm) | Reference |
|---|---|---|---|---|
| Chlamydomonas reinhardtii | Bioreducing agent: EPS; CAgNO3 1.25 mM; tsynthesis = 24 h; under illumination | Spherical | 7.5 | [39] |
| Chlorella pyrenoidosa | Bioreducing agent: EPS; CAgNO3 3.5 mM; T = 90 °C; pH = 8.0; tsynthesis = 60 min | Spherical | 5–15 | [36] |
| Chlorella vulgaris | Bioreducing agent: cell-free extract; CAgNO3 3.0 mM; T ambient; AgNO3 solution to cell-free extract ratio 8:2 (v/v); pH = 12.0; tsynthesis = 24 h | Spherical | 55.06 | [34] |
| Coelastrella aeroterrestrica | Bioreducing agent: aqueous cell extract; CAgNO3 1.0 mM; T ambient; AgNO3 solution to aqueous cell extract ratio 90:10 (v/v); tsynthesis = 24 h; under illumination | Hexagonal | 14.5 | [38] |
| Coelastrum astroideum, Desmodesmus armatus, Cosmarium punctulatum Klebsormidium flaccidum/ Synechococcus elongatus Microcystis aeruginosa | Bioreducing agent: aqueous cell extract; CAgNO3 1.0 mM; T ambient; AgNO3 solution to aqueous cell extract ratio 20:10 (v/v) tsynthesis = 20 h | Spherical | 1.8–5.4/2.3–2.6 | [37] |
| Cylindrospermum stagnale NCCU | Bioreducing agent: aqueous cell extract; CAgNO3 1.0 mM; T = 30 °C; tsynthesis = 250 h; under illumination | Pentagonal | 38–40 | [41] |
| Cystophora moniliformis (brown marine algae) | Bioreducing agent: aqueous extract; CAgNO3 1.0 mM; T = 65 °C; AgNO3 solution to aqueous cell extract ratio 90:10 (v/v); tsynthesis = 30 min | Spherical | 50–100 | [48] |
| Dunaliella salina | Bioreducing agent: aqueous cell extract; CAgNO3 1.0 mM; T ambient; AgNO3 solution to aqueous cell extract ratio 50:1 (v/v); tsynthesis = 15 min | Spherical | 35 | [40] |
| Dunaliella salina | Bioreducing agent: aqueous cell extract; CAgNO3 4.0 mM; pH = 7.0; T = 38 °C; AgNO3 solution to aqueous cell extract ratio 95:5 (v/v); tsynthesis = 35 min; under bright sunlight | Spherical | 1–30 | [42] |
| Hapalosiphon fontinalis NCCU-339 | Bioreducing agent: aqueous cell extract; CAgNO3 1.0 mM; T = 30 °C; tsynthesis = 270 h, under illumination | Triangular | 50 | [41] |
| Microchaete sp. NCCU-342 | Bioreducing agent: aqueous cell extract; CAgNO3 1.0 mM; T = 30 °C; tsynthesis = 30 h, under illumination | Spherical | 40 | [41] |
| Neochloris oleoabundans | Bioreducing agent: aqueous cell extract; CAgNO3 0.8 mM; pH = 5.0; T = 27 °C; tsynthesis = 6 h, under illumination | Quasi-spherical | 16.63 | [43] |
| Oscillatoria limnetica | Bioreducing agent: aqueous cell extract; CAgNO3 0.5 mM; pH = 6.7; T = 35 °C; AgNO3 solution to aqueous cell extract ratio 7:3 (v/v); tsynthesis = 48 h | Spherical | 3.30–17.97 | [49] |
| Phormidium sp. NCCU-104 | Bioreducing agent: aqueous cell extract; CAgNO3 1.0 mM; T = 30 °C; tsynthesis = 96 h; under illumination | Cubic | 48 | [41] |
| Haematococcus pluvialis | Bioreducing agent: CFS; CAgNO3 1.0 mM; T = 55 °C; pH = 11.0; tsynthesis = 6 h; under illumination | Quasi-spherical | 30–50 | Present study |
| Experimental Conditions | PDI | Zeta Potential (mV) |
|---|---|---|
| pH | ||
| 5 | 0.335 | −26.4 ± 5.9 |
| 7 | 0.291 | −26.7 ± 8.1 |
| 8 | 0.361 | −28.5 ± 6.7 |
| 9 | 0.258 | −28.6 ± 6.3 |
| 11 | 0.177 | −18.2 ± 5.2 |
| T (°C) | ||
| 25 | 0.322 | −30.5 ± 6.8 |
| 35 | 0.377 | −30.3 ± 6.5 |
| 45 | 0.341 | −32.9 ± 6.5 |
| 55 | 0.418 | −20.6 ± 4.6 |
| CAgNO3 (mM) | ||
| 1 | 0.377 | −30.5 ± 5.95 |
| 2 | 0.377 | −33.0 ± 7.95 |
| 3 | 0.31 | −32.7 ± 5.22 |
| 4 | 0.329 | −26.4 ± 5.85 |
| 5 | 0.356 | −22.9 ± 5.00 |
| AgNO3 Aqueous Solution tο Cell-Free Supernatant Ratio (v/v) | ||
| 95/5 | 0.313 | −34.1 ± 9.31 |
| 90/10 | 0.322 | −30.5 ± 6.84 |
| 85/15 | 0.332 | −29.0 ± 5.50 |
| Stirring (rpm) | ||
| Stirring at 180 rpm for 15 min followed by stirring at 80 rpm | 0.321 | −31.0 ± 7.33 |
| Stirring at 180 rpm for 15 min followed by static conditions | 0.293 | −35.4 ± 7.83 |
| Static conditions | 0.372 | −35.9 ± 7.06 |
| Continuous stirring at 180 rpm | 0.270 | −26.4 ± 4.70 |
| Under optimal conditions | 0.226 | −40.4 ± 8.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savvidou, M.G.; Kontari, E.; Kalantzi, S.; Mamma, D. Green Synthesis of Silver Nanoparticles Using the Cell-Free Supernatant of Haematococcus pluvialis Culture. Materials 2024, 17, 187. https://doi.org/10.3390/ma17010187
Savvidou MG, Kontari E, Kalantzi S, Mamma D. Green Synthesis of Silver Nanoparticles Using the Cell-Free Supernatant of Haematococcus pluvialis Culture. Materials. 2024; 17(1):187. https://doi.org/10.3390/ma17010187
Chicago/Turabian StyleSavvidou, Maria G., Evgenia Kontari, Styliani Kalantzi, and Diomi Mamma. 2024. "Green Synthesis of Silver Nanoparticles Using the Cell-Free Supernatant of Haematococcus pluvialis Culture" Materials 17, no. 1: 187. https://doi.org/10.3390/ma17010187
APA StyleSavvidou, M. G., Kontari, E., Kalantzi, S., & Mamma, D. (2024). Green Synthesis of Silver Nanoparticles Using the Cell-Free Supernatant of Haematococcus pluvialis Culture. Materials, 17(1), 187. https://doi.org/10.3390/ma17010187