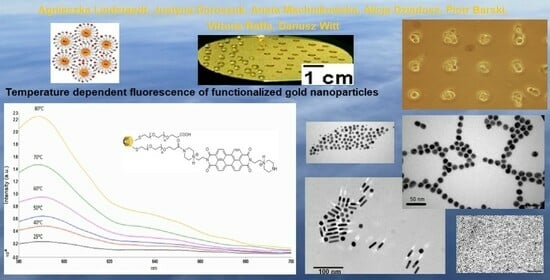
Effects Induced by the Temperature and Chemical Environment on the Fluorescence of Water-Soluble Gold Nanoparticles Functionalized with a Perylene-Derivative Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of N,N′-bis(2-(1-Piperazino)ethyl)-3,4,9,10-perylenetetracarboxylic Acid Diimide Dihydrochloride (PZPER)
2.2. Preparation of Gold Nanoparticles and Nanorods
2.3. Preparation of Functionalized Gold Nanoparticles
2.4. Determination of Size and Zeta Potential
2.5. Transmission Electron Microscopy (TEM)
2.6. UV–Vis and Fluorescence Measurement
3. Results and Discussion
3.1. Effects Induced by the Temperature and Buffers on the PZPER Fluorescence
3.2. Functionalization of AuNPs with PZPER
3.3. Functionalization of AuNRs with PZPER
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogle, M.M.; Smith McWilliams, A.D.; Jiang, B.; Martí, A.A. Latest trends in temperature sensing by molecular probes. ChemPhotoChem 2020, 4, 255–270. [Google Scholar] [CrossRef]
- Su, S.; Wei, J.; Zhang, K.; Qiu, J.; Wang, S. Thermo- and pH responsive fluorescence behaviors of sulfur-functionalized detonation nanodiamond-poly(N-isopropylacrylamide). Colloid Polym. Sci. 2015, 293, 1299–1305. [Google Scholar] [CrossRef]
- Arai, S.; Suzuki, M.; Park, S.-J.; Yoo, J.S.; Wang, L.; Kang, N.-Y.; Ha, H.-H.; Chang, Y.-T. Mitochondria-targeted fluorescent thermometer monitors intracellular temperature gradient. Chem. Commun. 2015, 51, 8044–8047. [Google Scholar] [CrossRef]
- Mirenda, M.; Levi, V.; Bossi, M.L.; Bruno, L.; Bordoni, A.V.; Regazzoni, A.E.; Wolosiuk, A. Temperature response of luminescent tris(bipyridine)Ruthenium(II)-doped silica nanoparticles. J. Colloid Interface Sci. 2013, 392, 96–101. [Google Scholar] [CrossRef]
- Ke, G.; Wang, C.; Ge, Y.; Zheng, N.; Zhu, Z.; Yang, C.J. l-DNA Molecular beacon: A safe, stable, and accurate intracellular nanothermometer for temperature sensing in living cells. J. Am. Chem. Soc. 2012, 134, 18908–18911. [Google Scholar] [CrossRef] [PubMed]
- Sadhanala, H.K.; Senapati, S.; Nanda, K.K. Temperature sensing using sulfur-doped carbon nanoparticles. Carbon 2018, 133, 200–208. [Google Scholar] [CrossRef]
- Jonstrup, A.T.; Fredsøe, J.; Andersen, A.H. DNA Hairpins as temperature switches, thermometers and ionic detectors. Sensors 2013, 13, 5937–5944. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, X.; Wang, D.; Hu, X.; Liu, T.; Zhang, G.; Liu, S. Ultrasensitive ratiometric fluorescent pH and temperature probes constructed from dye-labeled thermoresponsive double hydrophilic block copolymers. J. Mater. Chem. 2011, 21, 19030–19038. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Hu, R.; Xu, J.; Wang, S.; Li, S.; Li, T.; Yamg, G. Intracellular fluorescent temperature probe based on triarylboron substituted poly N-isopropylacrylamide and energy transfer. Anal. Chem. 2015, 87, 3694–3698. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zink, J.I. Taking the temperature of the interiors of magnetically heated nanoparticles. ACS Nano 2014, 8, 5199–5207. [Google Scholar] [CrossRef]
- Figueroa, I.D.; El Baraka, M.; Quiñones, E.; Rosario, O.; Deumie, M.A. Fluorescent temperature probe based on the association between the excited states of 4-(N,N-dimethylamino)-benzonitrile and β-cyclodextrin. Anal. Chem. 1998, 70, 3974–3977. [Google Scholar] [CrossRef]
- Pietsch, C.; Vollrath, A.; Hoogenboom, R.; Schubert, U.S. A fluorescent thermometer based on a pyrene-labeled thermoresponsive polymer. Sensors 2010, 10, 7979–7990. [Google Scholar] [CrossRef]
- Brandão-Silva, A.C.; Gomes, M.A.; Macedo, Z.S.; Avila, J.F.M.; Rodrigues, J.J., Jr.; Alencar, M.A.R.C. Multiwavelength fluorescence intensity ratio nanothermometry: High sensitivity over a broad temperature range. J. Phys. Chem. C 2018, 122, 20459–20468. [Google Scholar] [CrossRef]
- Xu, M.; Zou, X.; Su, Q.; Yuan, W.; Cao, C.; Wang, Q.; Zhu, X.; Feng, W.; Li, F. Ratiometric nanothermometer in vivo based on triplet sensitized upconversion. Nat. Commun. 2018, 9, 2698. [Google Scholar] [CrossRef]
- Xu, F.; Ba, Z.; Zheng, Y.; Wang, Y.; Hu, M.; Xu, X.; Wang, J.; Zhang, Z. Rare-Earth-doped optical nanothermometer in visible and near-infrared regions. J. Mater. Sci. 2018, 53, 15107–15117. [Google Scholar] [CrossRef]
- Quintanilla, M.; Zhang, Y.; Liz-Marzan, L.M. Subtissue plasmonic heating monitored with CaF2:Nd3+,Y3+ Nanothermometers in the second biological window. Chem. Mater. 2018, 30, 2819–2828. [Google Scholar] [CrossRef]
- Okabe, K.; Inada, N.; Gota, C.; Harada, Y.; Funatsu, T.; Uchiyama, S. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Commun. 2012, 3, 705. [Google Scholar] [CrossRef] [PubMed]
- Donner, J.S.; Thompson, S.A.; Kreuzer, M.P.; Baffou, G.; Quidant, R. Mapping intracellular temperature using green fluorescent protein. Nano Lett. 2012, 12, 2107–2111. [Google Scholar] [CrossRef] [PubMed]
- Homma, M.; Takei, Y.; Murata, A.; Inoue, T.; Takeoka, S. A ratiometric fluorescent molecular probe for visualization of mitochondrial temperature in living cells. Chem. Commun. 2015, 51, 6194–6197. [Google Scholar]
- Ren, M.; Brites, C.D.S.; Bao, S.-S.; Ferreira, R.A.S.; Zheng, L.-M.; Carlos, L.D. A cryogenic luminescent ratiometric thermometer based on a lanthanide phosphonate dimer. J. Mater. Chem. C 2015, 3, 8480–8484. [Google Scholar] [CrossRef]
- Feng, J.; Tian, K.; Hu, D.; Wang, S.; Li, S.; Zeng, Y.; Li, Y.; Yang, G. Triarylboron-based fluorescent thermometer: Sensitive over a wide temperature range. Angew. Chem. Int. Ed. 2011, 50, 8072–8076. [Google Scholar] [CrossRef]
- Chi, W.; Yin, W.; Qi, Q.; Qiao, Q.; Lin, Y.; Zhu, Z.; Vijayan, S.; Hashimoto, M.; Udayakumar, G.; Xu, Z.; et al. Ground-state conformers enable bright single-fluorophore ratiometric thermometers with positive temperature coefficients. Mater. Chem. Front. 2017, 1, 2383–2390. [Google Scholar] [CrossRef]
- Ibarra-Rodríguez, M.; Muñoz-Flores, B.M.; Dias, H.V.R.; Sánchez, M.; Gomez-Treviño, A.; Santillan, R.; Farfán, N.; Jiménez-Pérez, V.M. Fluorescent molecular rotors of organoboron compounds from Schiff bases: Synthesis, viscosity, reversible thermochromism, cytotoxicity, and bioimaging cells. J. Org. Chem. 2017, 82, 2375–2385. [Google Scholar] [CrossRef]
- Shi, M.; Xu, C.; Yang, Z.; Wang, L.; Tan, S.; Xu, G. Tuning of reversible thermochromic properties of salicylaldehyde Schiff bases through the substitution of methoxy and nitro groups. J. Mol. Struct. 2019, 1182, 72–78. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Chen, L.; Xiong, Y.; Yin, G. Cyano-functionalized 1,4-bis((E)-2-(1H-indol-3-yl)vinyl)benzenes with aggregation induced emission characteristic and diverse thermochromic behaviour. Dyes Pigm. 2016, 126, 194–201. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, Y.; Dimg, Y.; Meng, Y.; Zeng, Z.; Cabanetos, C.; Zhou, G.; Gao, J.; Liu, J.; Roncali, J. Mechanofluorochromic and thermochromic properties of simple tetraphenylethylene derivatives with fused fluorine containing 1,4-dioxocane rings. Dyes Pigm. 2017, 146, 323–330. [Google Scholar] [CrossRef]
- Yu, B.; Liu, D.; Wang, Y.; Zhang, T.; Zhang, Y.-M.; Li, M.; Zhang, S.X.-A. A solid-state emissive and solvatofluorochromic fluorophore and its application in high-contrast, fast, and repeatable thermochromic blends. Dyes Pigm. 2019, 163, 412–419. [Google Scholar] [CrossRef]
- Arai, S.; Lee, S.-C.; Zhai, D.; Suzuki, M.; Chang, Y.T. A molecular fluorescent probe for targeted visualization of temperature at the endoplasmic reticulum. Sci. Rep. 2015, 4, 6701. [Google Scholar] [CrossRef]
- Khattab, T.A.; Tiu, B.D.B.; Adas, S.; Bunge, S.D.; Advincula, R.C. Solvatochromic, thermochromic and pH-sensory DCDHF hydrazone molecular switch: Response to alkaline analytes. RSC Adv. 2016, 6, 102296–102305. [Google Scholar] [CrossRef]
- Belfield, K.D.; Bondar, M.V.; Przhonska, O.V.; Schafer, K.J. Photophysical characterization of 2,9-bis(7-benzothiazole-9,9′-didecylfluoren-2-yl)perylene diimide: A new standard for steady-state fluorescence anisotropy. J. Photochem. Photobiol. A Chem. 2002, 151, 7–11. [Google Scholar] [CrossRef]
- Icil, H.; Arslan, E. Synthesis and spectroscopic properties of highly pure perylene fluorescent dyes. Spect. Lett. 2001, 34, 355–363. [Google Scholar] [CrossRef]
- Kalinin, S.; Speckbacher, M.; Langhals, H.; Johansson, L.B.A. A new and versatile fluorescence standard for quantum yield determination. Phys. Chem. Chem. Phys. 2001, 3, 172–174. [Google Scholar] [CrossRef]
- Oh, J.H.; Sun, Y.-S.; Schmidt, R.; Toney, M.F.; Nordlund, D.; Konemann, M.; Wurthner, F.; Bao, Z. Interplay between energetic and kinetic factors on the ambient stability of n-channel organic transistors based on perylene diimide derivatives. Chem. Mater. 2009, 21, 5508–5518. [Google Scholar] [CrossRef]
- Weitz, R.T.; Amsharov, K.; Zschieschang, U.; Burghard, M.; Jansen, M.; Kelsch, M.; Rhamati, B.; van Aken, P.A.; Kern, K.; Klauk, H. The importance of grain boundaries for the time-dependent mobility degradation in organic thin-film transistors. Chem. Mater. 2009, 21, 4949–4954. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, Y.; Di, C.; Wang, Y.; Sun, X.; Guo, Y.; Zheng, J.; Wu, W.; Ye, S.; Yu, G. Improvements in stability and performance of N,N′-dialkyl perylene diimide-based n-type thin-film transistors. Adv. Mater. 2009, 21, 1631–1635. [Google Scholar] [CrossRef]
- Li, X.-Q.; Zhang, X.; Ghosh, S.; Wuerthner, F. Highly fluorescent lyotropic mesophases and organogels based on J-aggregates of core-twisted perylene bisimide dyes. Chem. Eur. J. 2008, 14, 8074–8078. [Google Scholar] [CrossRef] [PubMed]
- Wicklein, A.; Kohn, P.; Ghazaryan, L.; Thurn-Albrecht, T.; Thelakkat, M. Synthesis and structure elucidation of discotic liquid crystalline perylene imide benzimidazole. Chem. Commun. 2010, 46, 2328–2330. [Google Scholar] [CrossRef] [PubMed]
- Wicklein, A.; Lang, A.; Muth, M.; Thelakkat, M. Swallow-tail substituted liquid crystalline perylene bisimides: Synthesis and thermotropic properties. J. Am. Chem. Soc. 2009, 131, 14442–14453. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, N.R.; Wang, W.; Alloway, D.M.; Placencia, D.; Ratcliff, E.; Brumbach, M. Organic/organic′ heterojunctions: Organic light emitting diodes and organic photovoltaic devices. Macromol. Rapid Commun. 2009, 30, 717–731. [Google Scholar] [CrossRef]
- Imahori, H.; Umeyama, T.; Ito, S. Large π-aromatic molecules as potential sensitizers for highly efficient dye-sensitized solar cells. Acc. Chem. Res. 2009, 42, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Oh, J.H.; Sun, Y.-S.; Deppisch, M.; Krause, A.-M.; Radacki, K.; Braunschweig, H.; Konemann, M.; Erk, P.; Bao, Z.; et al. High-performance air-stable n-channel organic thin film transistors based on halogenated perylene bisimide semiconductors. J. Am. Chem. Soc. 2009, 131, 6215–6228. [Google Scholar] [CrossRef] [PubMed]
- Tolkki, A.; Vuorimaa, E.; Chukharev, V.; Lemmetyinen, H.; Ihalainen, P.; Peltonen, J.; Dehm, V.; Wurthner, F. Langmuir−Schaeffer films from a π−π stacking perylenediimide dye: Organization and charge transfer properties. Langmuir 2010, 26, 6630–6637. [Google Scholar] [CrossRef] [PubMed]
- Wuerthner, F. Perylene bisimide dyes as versatile building blocks for functional supramolecular architectures. Chem. Commun. 2004, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Langhals, H.; Saulich, S. Bichromophoric perylene derivatives: Energy transfer from non-fluorescent chromophores. Chem. Eur. J. 2002, 8, 5630–5643. [Google Scholar] [CrossRef] [PubMed]
- Langhals, H.; Kirner, S. Novel fluorescent dyes by the extension of the core of perylenetetracarboxylic bisimides. Eur. J. Org. Chem. 2000, 2000, 365–380. [Google Scholar] [CrossRef]
- Franceschin, M.; Lombardo, C.M.; Pascucci, E.; D’Ambrosio, D.; Micheli, E.; Bianco, A.; Ortaggia, G.; Savino, M. The number and distances of positive charges of polyamine side chains in a series of perylene diimides significantly influence their ability to induce G-quadruplex structures and inhibit human telomerase. Bioorg. Med. Chem. 2008, 16, 2292–2304. [Google Scholar] [CrossRef]
- Tang, T.; Peneva, K.; Muellen, K.; Webber, S.E. Photophysics of water soluble perylene diimides in surfactant solutions. J. Phys. Chem. A 2007, 111, 10609–10614. [Google Scholar] [CrossRef]
- Tang, T.; Herrmann, A.; Peneva, K.; Muellen, K.; Webber, S.E. Energy transfer in molecular layer-by-layer films of water-soluble perylene diimides. Langmuir 2007, 23, 4623–4628. [Google Scholar] [CrossRef]
- Franceschin, M.; Pascucci, E.; Alvino, A.; D’Ambrosio, D.; Bianco, A.; Ortaggia, G.; Savino, M. New highly hydrosoluble and not self-aggregated perylene derivatives with three and four polar side-chains as G-quadruplex telomere targeting agents and telomerase inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 2515–2522. [Google Scholar] [CrossRef]
- Alvino, A.; Franceschin, M.; Cefaro, C.; Borioni, S.; Ortaggi, G.; Bianco, A. Synthesis and spectroscopic properties of highly water-soluble perylene derivatives. Tetrahedron 2007, 63, 7858–7865. [Google Scholar] [CrossRef]
- Tang, T.; Qu, J.; Muellen, K.; Webber, S.E. Molecular layer-by-layer self-assembly of water-soluble perylene diimides through π−π and electrostatic interactions. Langmuir 2006, 22, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Donati, F.; Pucci, A.; Ruggeri, G. Temperature and chemical environment effects on the aggregation extent of water soluble perylenedye into vinyl alcohol-containing polymers. Phys. Chem. Chem. Phys. 2009, 11, 6276–6282. [Google Scholar] [CrossRef] [PubMed]
- Rahme, K.; Chen, L.; Hobbs, R.G.; Morris, M.A.; O’Driscoll, C.; Holmes, J.D. PEGylated gold nanoparticles: Polymer quantification as a function of PEG lengths and nanoparticle dimensions. RSC Adv. 2013, 3, 6085–6094. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindstaedt, A.; Doroszuk, J.; Machnikowska, A.; Dziadosz, A.; Barski, P.; Raffa, V.; Witt, D. Effects Induced by the Temperature and Chemical Environment on the Fluorescence of Water-Soluble Gold Nanoparticles Functionalized with a Perylene-Derivative Dye. Materials 2024, 17, 1097. https://doi.org/10.3390/ma17051097
Lindstaedt A, Doroszuk J, Machnikowska A, Dziadosz A, Barski P, Raffa V, Witt D. Effects Induced by the Temperature and Chemical Environment on the Fluorescence of Water-Soluble Gold Nanoparticles Functionalized with a Perylene-Derivative Dye. Materials. 2024; 17(5):1097. https://doi.org/10.3390/ma17051097
Chicago/Turabian StyleLindstaedt, Agnieszka, Justyna Doroszuk, Aneta Machnikowska, Alicja Dziadosz, Piotr Barski, Vittoria Raffa, and Dariusz Witt. 2024. "Effects Induced by the Temperature and Chemical Environment on the Fluorescence of Water-Soluble Gold Nanoparticles Functionalized with a Perylene-Derivative Dye" Materials 17, no. 5: 1097. https://doi.org/10.3390/ma17051097
APA StyleLindstaedt, A., Doroszuk, J., Machnikowska, A., Dziadosz, A., Barski, P., Raffa, V., & Witt, D. (2024). Effects Induced by the Temperature and Chemical Environment on the Fluorescence of Water-Soluble Gold Nanoparticles Functionalized with a Perylene-Derivative Dye. Materials, 17(5), 1097. https://doi.org/10.3390/ma17051097