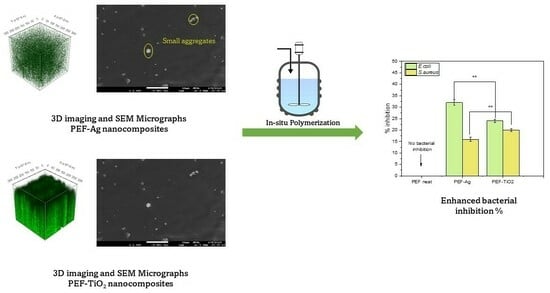
Fabrication of Poly(ethylene furanoate)/Silver and Titanium Dioxide Nanocomposites with Improved Thermal and Antimicrobial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.2.1. Intrinsic Viscosity ([η]) and Molecular Weight (Mn) Determination
2.2.2. Color Analysis
2.2.3. Scanning Electron Microscopy (SEM)
2.2.4. Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.2.5. Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS)
2.2.6. Differential Scanning Calorimetry (DSC)
2.2.7. X-ray Diffraction (XRD)
2.2.8. Broadband Dielectric Spectroscopy (BDS)
2.2.9. Contact Angle (CA) Analysis
2.2.10. Antibacterial Studies
3. Results
3.1. Fabrication and Color Measurements of PEF and Respective Nanocomposites
3.2. SEM-EDX
3.3. ATR-FTIR Spectroscopy
3.4. ToF-SIMS Depth Profiling Analysis of the Nanocomposites
3.5. Thermal Properties and Crystallinity
3.6. Broadband Dielectric Spectroscopy
3.7. CA Analysis
3.8. Antimicrobial Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial Nanocellulose—A Biobased Polymer for Active and Intelligent Food Packaging Applications: Recent Advances and Developments. Polymers 2020, 12, 2209. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial Agents and Packaging Systems in Antimicrobial Active Food Packaging: An Overview of Approaches and Interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Hamzehlou, S.; Aboudzadeh, M.A. Special Issue on “Multifunctional Hybrid Materials Based on Polymers: Design and Performance”. Processes 2021, 9, 1448. [Google Scholar] [CrossRef]
- Aboudzadeh, M.A.; Iturrospe, A.; Arbe, A.; Grzelczak, M.; Barroso-Bujans, F. Cyclic Polyethylene Glycol as Nanoparticle Surface Ligand. ACS Macro Lett. 2020, 9, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.; Visser, H.A.; Dias, A.S.; Harvey, C.; Gruter, G.-J.M. The Road to Bring FDCA and PEF to the Market. Polymers 2022, 14, 943. [Google Scholar] [CrossRef]
- Gabirondo, E.; Melendez-Rodriguez, B.; Arnal, C.; Lagaron, J.M.; Martínez de Ilarduya, A.; Sardon, H.; Torres-Giner, S. Organocatalyzed Closed-Loop Chemical Recycling of Thermo-Compressed Films of Poly(Ethylene Furanoate). Polym. Chem. 2021, 12, 1571–1580. [Google Scholar] [CrossRef]
- Zhu, C.; Yin, J.; Zhang, Z.; Shi, F. Bio-Based Poly(Ethylene Furanoate)/ZnO Transparent Thin Films with Improved Water Vapor Barrier and Antibacterial Properties for Food Packaging Application. Mater. Res. Express 2022, 9, 115304. [Google Scholar] [CrossRef]
- Zhu, C.; Ye, D.; Zhou, T.; Cui, Y.; Yin, J. High-Antimicrobial Gallium-Doped Zinc Oxide Thin Films on Bio-Based Poly(Ethylene Furanoate) Substrates for Food Packaging Application. Membranes 2023, 13, 239. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Xanthopoulou, E.; Finšgar, M.; Zemljič, L.F.; Klonos, P.A.; Kyritsis, A.; Koltsakidis, S.; Tzetzis, D.; Lambropoulou, D.A.; Baciu, D.; et al. Synthesis of Poly(Ethylene Furanoate) Based Nanocomposites by In Situ Polymerization with Enhanced Antibacterial Properties for Food Packaging Applications. Polymers 2023, 15, 4502. [Google Scholar] [CrossRef]
- Stanley, J.; John, A.; Pušnik Črešnar, K.; Fras Zemljič, L.; Lambropoulou, D.A.; Bikiaris, D.N. Active Agents Incorporated in Polymeric Substrates to Enhance Antibacterial and Antioxidant Properties in Food Packaging Applications. Macromol 2022, 3, 1–27. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A Concise Guide to Active Agents for Active Food Packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Ashfaq, A.; Khursheed, N.; Fatima, S.; Anjum, Z.; Younis, K. Application of Nanotechnology in Food Packaging: Pros and Cons. J. Agric. Food Res. 2022, 7, 100270. [Google Scholar] [CrossRef]
- Otoni, C.G.; Espitia, P.J.P.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in Antimicrobial Food Packaging Systems: Emitting Sachets and Absorbent Pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Zou, L.; Brody, A.L.; Li, Z.; Qazi, I.M.; Pavase, T.R.; Lv, L. A Comprehensive Review on the Application of Active Packaging Technologies to Muscle Foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Roy, S.; Ghosh, T.; Biswas, D.; Rhim, J.-W. Antimicrobial Nanofillers Reinforced Biopolymer Composite Films for Active Food Packaging Applications—A Review. Sustain. Mater. Technol. 2022, 32, e00353. [Google Scholar] [CrossRef]
- Qi, Y.; Xiang, B.; Tan, W.; Zhang, J. Hydrophobic Surface Modification of TiO2 Nanoparticles for Production of Acrylonitrile-Styrene-Acrylate Terpolymer/TiO2 Composited Cool Materials. Appl. Surf. Sci. 2017, 419, 213–223. [Google Scholar] [CrossRef]
- Mendes, S.F.; Costa, C.M.; Caparros, C.; Sencadas, V.; Lanceros-Méndez, S. Effect of Filler Size and Concentration on the Structure and Properties of Poly(Vinylidene Fluoride)/BaTiO3 Nanocomposites. J. Mater. Sci. 2012, 47, 1378–1388. [Google Scholar] [CrossRef]
- Kumar, S.; Shukla, A.; Baul, P.P.; Mitra, A.; Halder, D. Biodegradable Hybrid Nanocomposites of Chitosan/Gelatin and Silver Nanoparticles for Active Food Packaging Applications. Food Packag. Shelf Life 2018, 16, 178–184. [Google Scholar] [CrossRef]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennis, T.T.; Wacławek, S.; Černík, M.; Padil, V.V.T. Chitosan/Gelatin/Silver Nanoparticles Composites Films for Biodegradable Food Packaging Applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Z.; Liu, Y.; Zheng, X.; Pei, Y.; Tang, K. Soluble Soybean Polysaccharide Films Containing In-Situ Generated Silver Nanoparticles for Antibacterial Food Packaging Applications. Food Packag. Shelf Life 2022, 31, 100800. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver Nanoparticles Are Broad-Spectrum Bactericidal and Virucidal Compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef]
- Azlin-Hasim, S.; Cruz-Romero, M.C.; Morris, M.A.; Cummins, E.; Kerry, J.P. Effects of a Combination of Antimicrobial Silver Low Density Polyethylene Nanocomposite Films and Modified Atmosphere Packaging on the Shelf Life of Chicken Breast Fillets. Food Packag. Shelf Life 2015, 4, 26–35. [Google Scholar] [CrossRef]
- Chadha, U.; Bhardwaj, P.; Selvaraj, S.K.; Arasu, K.; Praveena, S.; Pavan, A.; Khanna, M.; Singh, P.; Singh, S.; Chakravorty, A.; et al. Current Trends and Future Perspectives of Nanomaterials in Food Packaging Application. J. Nanomater. 2022, 2022, 2745416. [Google Scholar] [CrossRef]
- Othman, S.H.; Abd Salam, N.R.; Zainal, N.; Kadir Basha, R.; Talib, R.A. Antimicrobial Activity of TiO2 Nanoparticle-Coated Film for Potential Food Packaging Applications. Int. J. Photoenergy 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and Characterization of Chitosan-Titanium Dioxide Nanocomposite Film as Ethylene Scavenging and Antimicrobial Active Food Packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- ISO22196; Plastics—Measurement of Antibacterial Activity on Plastics Surfaces. Organization for Standardization: Geneva, Switzerland, 2007.
- Kim, E.S.; Kim, S.H.; Lee, C.H. Electrospinning of Polylactide Fibers Containing Silver Nanoparticles. Macromol. Res. 2010, 18, 215–221. [Google Scholar] [CrossRef]
- Stanley, J.; Terzopoulou, Z.; Klonos, P.A.; Zamboulis, A.; Xanthopoulou, E.; Koltsakidis, S.; Tzetzis, D.; Zemljič, L.F.; Lambropoulou, D.A.; Kyritsis, A.; et al. Effect of Monomer Type on the Synthesis and Properties of Poly(Ethylene Furanoate). Polymers 2023, 15, 2707. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, L.; Rajini, N.; Nellaiah, H.; Kathiresan, T.; Jawaid, M.; Varada Rajulu, A. Experimental Investigation of Cellulose/Silver Nanocomposites Using In Situ Generation Method. J. Polym. Environ. 2017, 25, 1021–1032. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Maleki, M.; Eghbaljoo-Gharehgheshlaghi, H.; Khezerlou, A.; Mohammadian, E.; Liu, Q.; Jafari, S.M. Titanium Dioxide Nanoparticles as Multifunctional Surface-Active Materials for Smart/Active Nanocomposite Packaging Films. Adv. Colloid Interface Sci. 2022, 300, 102593. [Google Scholar] [CrossRef]
- Grothe, J.; Kaskel, S.; Leuteritz, A. Nanocomposites and Hybrid Materials. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 177–209. [Google Scholar]
- Alizadeh-Sani, M.; Moghaddas Kia, E.; Ghasempour, Z.; Ehsani, A. Preparation of Active Nanocomposite Film Consisting of Sodium Caseinate, ZnO Nanoparticles and Rosemary Essential Oil for Food Packaging Applications. J. Polym. Environ. 2021, 29, 588–598. [Google Scholar] [CrossRef]
- Peng, X.; Ding, E.; Xue, F. In Situ Synthesis of TiO2/Polyethylene Terephthalate Hybrid Nanocomposites at Low Temperature. Appl. Surf. Sci. 2012, 258, 6564–6570. [Google Scholar] [CrossRef]
- Nanayakkara, C.E.; Larish, W.A.; Grassian, V.H. Titanium Dioxide Nanoparticle Surface Reactivity with Atmospheric Gases, CO2, SO2, and NO2: Roles of Surface Hydroxyl Groups and Adsorbed Water in the Formation and Stability of Adsorbed Products. J. Phys. Chem. C 2014, 118, 23011–23021. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Zhang, J.; Yu, X.; Wu, Z. Influence of Surface Functional Groups on Deposition and Release of TiO2 Nanoparticles. Environ. Sci. Technol. 2017, 51, 7467–7475. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Yao, H.; Chen, C.; Lu, X.; Ding, P.; Wang, M.; Hua, X.; Shan, A. Chemical Reaction and Bonding Mechanism at the Polymer-Metal Interface. ACS Appl. Mater. Interfaces 2022, 14, 27383–27396. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Finšgar, M. Analytical Techniques for the Characterization of Bioactive Coatings for Orthopaedic Implants. Biomedicines 2021, 9, 1936. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.Z.; Papageorgiou, D.G.; Tsanaktsis, V.; Bikiaris, D.N. Synthesis of the Bio-Based Polyester Poly(Propylene 2,5-Furan Dicarboxylate). Comparison of Thermal Behavior and Solid State Structure with Its Terephthalate and Naphthalate Homologues. Polymer 2015, 62, 28–38. [Google Scholar] [CrossRef]
- Klonos, P.A.; Papadopoulos, L.; Papageorgiou, G.Z.; Kyritsis, A.; Pissis, P.; Bikiaris, D.N. Interfacial Interactions, Crystallization, and Molecular Dynamics of Renewable Poly(Propylene Furanoate) In Situ Filled with Initial and Surface Functionalized Carbon Nanotubes and Graphene Oxide. J. Phys. Chem. C 2020, 124, 10220–10234. [Google Scholar] [CrossRef]
- Sargsyan, A.; Tonoyan, A.; Davtyan, S.; Schick, C. The Amount of Immobilized Polymer in PMMA SiO2 Nanocomposites Determined from Calorimetric Data. Eur. Polym. J. 2007, 43, 3113–3127. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Klonos, P.A.; Kyritsis, A.; Tziolas, A.; Avgeropoulos, A.; Papageorgiou, G.Z.; Bikiaris, D.N. Interfacial interactions, crystallization and molecular mobility in nanocomposites of poly(lactic acid) filled with new hybrid inclusions based on graphene oxide and silica nanoparticles. Polymer 2019, 166, 1–12. [Google Scholar] [CrossRef]
- Stoclet, G.; Arias, A.; Yeniad, B.; De Vos, S. Relationships between Crystalline Structure and the Thermal Behavior of Poly(Ethylene 2,5-furandicarboxylate): An in Situ Simultaneous SAXS-WAXS Study. Polym. Eng. Sci. 2019, 59, 1667–1677. [Google Scholar] [CrossRef]
- Mankar, S.V.; Garcia Gonzalez, M.N.; Warlin, N.; Valsange, N.G.; Rehnberg, N.; Lundmark, S.; Jannasch, P.; Zhang, B. Synthesis, Life Cycle Assessment, and Polymerization of a Vanillin-Based Spirocyclic Diol toward Polyesters with Increased Glass-Transition Temperature. ACS Sustain. Chem. Eng. 2019, 7, 19090–19103. [Google Scholar] [CrossRef]
- Righetti, M.C.; Marchese, P.; Vannini, M.; Celli, A.; Tricoli, F.; Lorenzetti, C. Temperature-Induced Polymorphism in Bio-Based Poly(Propylene 2,5-Furandicarboxylate). Thermochim. Acta 2019, 677, 186–193. [Google Scholar] [CrossRef]
- Kaur, A.; Khanna, A.; Kaur, A.; Hirdesh; Gonzàlez-Barriuso, M.; Gonzàlez, F. Effects of Annealing on Density, Glass Transition Temperature and Structure of Tellurite, Silicate and Borate Glasses. J. Non-Cryst. Solids 2018, 500, 443–452. [Google Scholar] [CrossRef]
- Stoclet, G.; Gobius du Sart, G.; Yeniad, B.; de Vos, S.; Lefebvre, J.M. Isothermal Crystallization and Structural Characterization of Poly(Ethylene-2,5-Furanoate). Polymer 2015, 72, 165–176. [Google Scholar] [CrossRef]
- Kourtidou, D.; Klonos, P.A.; Papadopoulos, L.; Kyritsis, A.; Bikiaris, D.N.; Chrissafis, K. Molecular Mobility and Crystallization of Renewable Poly(Ethylene Furanoate) in Situ Filled with Carbon Nanotubes and Graphene Nanoparticles. Soft Matter 2021, 17, 5815–5828. [Google Scholar] [CrossRef] [PubMed]
- Dynamics of Composite Materials; Schönhals, A.; Szymoniak, P. (Eds.) Springer International Publishing: Cham, Swizerland, 2022; ISBN 978-3-030-89722-2. [Google Scholar]
- Genovese, L.; Soccio, M.; Lotti, N.; Munari, A.; Szymczyk, A.; Paszkiewicz, S.; Linares, A.; Nogales, A.; Ezquerra, T.A. Effect of Chemical Structure on the Subglass Relaxation Dynamics of Biobased Polyesters as Revealed by Dielectric Spectroscopy: 2,5-Furandicarboxylic Acid vs. Trans -1,4-Cyclohexanedicarboxylic Acid. Phys. Chem. Chem. Phys. 2018, 20, 15696–15706. [Google Scholar] [CrossRef]
- Soccio, M.; Martínez-Tong, D.E.; Guidotti, G.; Robles-Hernández, B.; Munari, A.; Lotti, N.; Alegria, A. Broadband Dielectric Spectroscopy Study of Biobased Poly(Alkylene 2,5-Furanoate)s’ Molecular Dynamics. Polymers 2020, 12, 1355. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, N.D.; Klonos, P.A.; Ioannidis, R.O.; Saranti, P.; Barmpalexis, P.; Kyritsis, A. Molecular mobility and thermal transitions study in renewable PLA-polyols star-like copolymers. Polymer 2024, 292, 126635. [Google Scholar] [CrossRef]
- Xing, K.; Tress, M.; Cao, P.-F.; Fan, F.; Cheng, S.; Saito, T.; Sokolov, A.P. The Role of Chain-End Association Lifetime in Segmental and Chain Dynamics of Telechelic Polymers. Macromolecules 2018, 51, 8561–8573. [Google Scholar] [CrossRef]
- Broadband Dielectric Spectroscopy; Kremer, F.; Schönhals, A. (Eds.) Springer: Berlin/Heidelberg, Germany, 2003; ISBN 978-3-642-62809-2. [Google Scholar]
- Črešnar, K.P.; Aulova, A.; Bikiaris, D.N.; Lambropoulou, D.; Kuzmič, K.; Zemljič, L.F. Incorporation of Metal-based Nanoadditives into the Pla Matrix: Effect of Surface Properties on Antibacterial Activity and Mechanical Performance of Pla Nanoadditive Films. Molecules 2021, 26, 4161. [Google Scholar] [CrossRef]
- Klonos, P.; Kulyk, K.; Borysenko, M.V.; Gun’ko, V.M.; Kyritsis, A.; Pissis, P. Effects of Molecular Weight below the Entanglement Threshold on Interfacial Nanoparticles/Polymer Dynamics. Macromolecules 2016, 49, 9457–9473. [Google Scholar] [CrossRef]
- Prakash, J.; Pivin, J.C.; Swart, H.C. Noble Metal Nanoparticles Embedding into Polymeric Materials: From Fundamentals to Applications. Adv. Colloid Interface Sci. 2015, 226, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Sun, C.; Bai, B. Molecular Dynamics Study on the Effect of Surface Hydroxyl Groups on Three-Phase Wettability in Oil-Water-Graphite Systems. Polymers 2017, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, D.; Kanmani, P. Study on Physical and Mechanical Properties of the Biopolymer/Silver Based Active Nanocomposite Films with Antimicrobial Activity. Carbohydr. Polym. 2019, 224, 115159. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. In Vivo Evaluation of Chitosan–PVP–Titanium Dioxide Nanocomposite as Wound Dressing Material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Guerrero Correa, M.; Martínez, F.B.; Streitt, C.; José Galotto, M. Antimicrobial Effect of Titanium Dioxide Nanoparticles. In Antimicrobial Resistance—A One Health Perspective; IntechOpen: London, UK, 2021. [Google Scholar]
- Xing, Y.; Li, X.; Zhang, L.; Xu, Q.; Che, Z.; Li, W.; Bai, Y.; Li, K. Effect of TiO2 Nanoparticles on the Antibacterial and Physical Properties of Polyethylene-Based Film. Prog. Org. Coat. 2012, 73, 219–224. [Google Scholar] [CrossRef]











| Sample | Active Agents (wt.%) | [η] (dL/g) | Mn (g/mol) |
|---|---|---|---|
| PEF neat | - | 0.43 | 8900 |
| PEF-Ag | Silver nanoparticles (1) | 0.47 | 10,200 |
| PEF-TiO2 | Titanium dioxide (1) | 0.45 | 9600 |
| Sample | L* | a* | b* | c* | h° | R | K/S |
|---|---|---|---|---|---|---|---|
| PEF neat | 91.02 | −2.17 | 7.78 | 8.57 | 108.60 | 47.14 (400 nm) | 0.4 |
| PEF-Ag | 61.67 | 4.12 | 25.44 | 25.56 | 81.78 | 10.76 (400 nm) | 3.7 |
| PEF-TiO2 | 73.88 | 6.08 | 27.15 | 23.12 | 76.25 | 14.63 (400 nm) | 2.5 |
| Melt-Fast Cooled Scan 1 | Melt-Slow Cooled Scan 2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Mn (g/mol) | Tg (oC) | Δcp (J/g∙K) | Tcc (oC) | Xcc (%) | Tm (oC) | Xm (%) | Tc (oC) | Xc (%) | Tg (oC) | Δcp (J/g∙K) | Tm (oC) | ΔHm (J/g) |
| PEF neat | 8.9 k | 82 | 0.46 | 174 | 5 | 203 | 6 | - | 0 | 82 | 0.46 | - | 0 |
| PEF-TiO2 | 8.9 k | 83 | 0.44 | 178 | 2 | 204 | 2 | - | 0 | 83 | 0.41 | - | 0 |
| PEF-Ag | 10.2 k | 85 | 0.46 | 187 | ~0 | 210 | ~0 | - | 0 | 85 | 0.49 | - | 0 |
| Samples | Tg (°C) | Tm1 (°C) | Tm2 (°C) | ΔHm − ΔHcc(J/g) | Xc a (%) | Xc b (%) |
|---|---|---|---|---|---|---|
| PEF neat | 82 | 175 | 201 | 40.18 | 29 | 31.5 |
| PEF-Ag | 86 | 179 | 206 | 37.90 | 27 | 28 |
| PEF-TiO2 | 82 | 180 | 198 | 35.77 | 26 | 30 |
| DSC Scan 1 | BDS Melted and Fast-Cooled | |||
|---|---|---|---|---|
| Sample | Mn (g/mol) | Tg (°C) | Tg, diel (°C) | fragility index mα |
| PEF neat | 8.9k | 82 | 75 | 100 |
| PEF-TiO2 | 8.9k | 83 | 75 | 101 |
| PEF-Ag | 10.2k | 85 | 69 | 95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanley, J.; Xanthopoulou, E.; Zemljič, L.F.; Klonos, P.A.; Kyritsis, A.; Lambropoulou, D.A.; Bikiaris, D.N. Fabrication of Poly(ethylene furanoate)/Silver and Titanium Dioxide Nanocomposites with Improved Thermal and Antimicrobial Properties. Materials 2024, 17, 1606. https://doi.org/10.3390/ma17071606
Stanley J, Xanthopoulou E, Zemljič LF, Klonos PA, Kyritsis A, Lambropoulou DA, Bikiaris DN. Fabrication of Poly(ethylene furanoate)/Silver and Titanium Dioxide Nanocomposites with Improved Thermal and Antimicrobial Properties. Materials. 2024; 17(7):1606. https://doi.org/10.3390/ma17071606
Chicago/Turabian StyleStanley, Johan, Eleftheria Xanthopoulou, Lidija Fras Zemljič, Panagiotis A. Klonos, Apostolos Kyritsis, Dimitra A. Lambropoulou, and Dimitrios N. Bikiaris. 2024. "Fabrication of Poly(ethylene furanoate)/Silver and Titanium Dioxide Nanocomposites with Improved Thermal and Antimicrobial Properties" Materials 17, no. 7: 1606. https://doi.org/10.3390/ma17071606
APA StyleStanley, J., Xanthopoulou, E., Zemljič, L. F., Klonos, P. A., Kyritsis, A., Lambropoulou, D. A., & Bikiaris, D. N. (2024). Fabrication of Poly(ethylene furanoate)/Silver and Titanium Dioxide Nanocomposites with Improved Thermal and Antimicrobial Properties. Materials, 17(7), 1606. https://doi.org/10.3390/ma17071606