Synthesis of Bio-Based Polyester Resins for Vat Photopolymerization 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Polyester Synthesis
- PEA-BIO-1: cyclohexanedimethanol, 1,3 propanediol, sebacic acid, succinic acid, hypophosphorous acid, phthalic acid anhydride.
- PEA-BIO-2 and PEA-BIO-3: isosorbide, 1,3 propanediol, sebacic acid, succinic acid, hypophosphorous acid, phthalic acid anhydride.
2.3. Preparation of Resin Formulations
2.4. Resin Characterization
2.5. Color Measurement
2.6. DLP 3D Printing
2.7. Characterization of Mechanical and Thermomechanical Properties
3. Results and Discussion
3.1. Polyester Synthesis
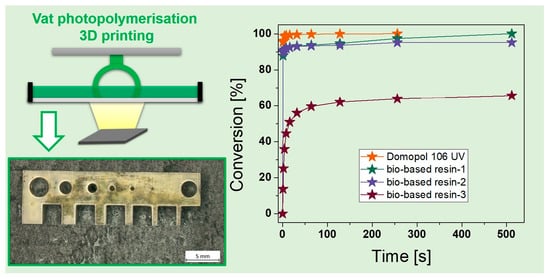
3.2. Additive Manufacturing
3.3. Mechanical and Thermomechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Voet, V.S.D.; Schnelting, G.H.M.; Xu, J.; Loos, K.; Folkersma, R.; Jager, J. Stereolithographic 3D Printing with Renewable Acrylates. J. Vis. Exp. 2018, 139, e58177. [Google Scholar] [CrossRef]
- Maines, E.M.; Porwal, M.K.; Ellison, C.J.; Reineke, T.M. Sustainable advances in SLA/DLP 3D printing materials and processes. Green Chem. 2021, 23, 6863–6897. [Google Scholar] [CrossRef]
- Voet, V.S.D.; Guit, J.; Loos, K. Sustainable Photopolymers in 3D Printing: A Review on Biobased, Biodegradable, and Recyclable Alternatives. Macromol. Rapid Commun. 2021, 42, e2000475. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.M.; Ali, Z. Fabrication Methods for Microfluidic Devices: An Overview. Micromachines 2021, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Fabbri, P.; Leoni, E.; Mazzanti, F.; Akbari, R.; Antonini, C. Additive manufacturing by digital light processing: A review. Prog. Addit. Manuf. 2023, 8, 331–351. [Google Scholar] [CrossRef]
- Pou, J.; Riveiro, A.; Davim, J.P.; Davim, J.P. Additive Manufacturing; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128184110. [Google Scholar]
- Skliutas, E.; Kasetaite, S.; Jonušauskas, L.; Ostrauskaite, J.; Malinauskas, M. Photosensitive naturally derived resins toward optical 3-D printing. Opt. Eng. 2018, 57, 041412. [Google Scholar] [CrossRef]
- Thrasher, C.J.; Schwartz, J.J.; Boydston, A.J. Modular Elastomer Photoresins for Digital Light Processing Additive Manufacturing. ACS Appl. Mater. Interfaces 2017, 9, 39708–39716. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, L.; Jian, M.; Mao, Y.; Yu, M.; Guo, X. EHMP-DLP: Multi-projector DLP with energy homogenization for large-size 3D printing. Rapid Prototyp. J. 2018, 24, 1500–1510. [Google Scholar] [CrossRef]
- Wu, Y.; Su, H.; Li, M.; Xing, H. Digital light processing-based multi-material bioprinting: Processes, applications, and perspectives. J. Biomed. Mater. Res. Part A 2023, 111, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Tian, X.; Song, X. Engineering materials with light: Recent progress in digital light processing based 3D printing. J. Mater. Chem. C 2020, 8, 13896–13917. [Google Scholar] [CrossRef]
- Ligon-Auer, S.C.; Schwentenwein, M.; Gorsche, C.; Stampfl, J.; Liska, R. Toughening of photo-curable polymer networks: A review. Polym. Chem. 2016, 7, 257–286. [Google Scholar] [CrossRef]
- Yang, E.; Miao, S.; Zhong, J.; Zhang, Z.; Mills, D.K.; Zhang, L.G. Bio-Based Polymers for 3D Printing of Bioscaffolds. Polym. Rev. 2018, 58, 668–687. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Guit, J.; Tavares, M.B.L.; Hul, J.; Ye, C.; Loos, K.; Jager, J.; Folkersma, R.; Voet, V.S.D. Photopolymer Resins with Biobased Methacrylates Based on Soybean Oil for Stereolithography. ACS Appl. Polym. Mater. 2020, 2, 949–957. [Google Scholar] [CrossRef]
- Lu, C.; Wang, C.; Yu, J.; Wang, J.; Chu, F. Two-Step 3 D-Printing Approach toward Sustainable, Repairable, Fluorescent Shape-Memory Thermosets Derived from Cellulose and Rosin. ChemSusChem 2020, 13, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Mohawk, D.; Jahani, B.; Wang, X.; Chen, Y.; Mahoney, A.; Zhu, J.Y.; Jiang, L. UV-Curable Cellulose Nanofiber-Reinforced Soy Protein Resins for 3D Printing and Conventional Molding. ACS Appl. Polym. Mater. 2020, 2, 4666–4676. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Ostrauskaite, J.; Skliutas, E.; Malinauskas, M. Photocross-linked polymers based on plant-derived monomers for potential application in optical 3D printing. J. Appl. Polym. Sci. 2020, 137, 2130. [Google Scholar] [CrossRef]
- Barkane, A.; Platnieks, O.; Jurinovs, M.; Kasetaite, S.; Ostrauskaite, J.; Gaidukovs, S.; Habibi, Y. UV-Light Curing of 3D Printing Inks from Vegetable Oils for Stereolithography. Polymers 2021, 13, 1195. [Google Scholar] [CrossRef]
- Briede, S.; Barkane, A.; Jurinovs, M.; Thakur, V.K.; Gaidukovs, S. Acrylation of biomass: A review of synthesis process: Know-how and future application directions. Curr. Opin. Green Sustain. Chem. 2022, 35, 100626. [Google Scholar] [CrossRef]
- Barkane, A.; Jurinovs, M.; Briede, S.; Platnieks, O.; Onufrijevs, P.; Zelca, Z.; Gaidukovs, S. Biobased Resin for Sustainable Stereolithography: 3D Printed Vegetable Oil Acrylate Reinforced with Ultra-Low Content of Nanocellulose for Fossil Resin Substitution. 3D Print. Addit. Manuf. 2022, 16, 171. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Guzmán, D.B.; González-Delgado, J.A.; Arteaga, J.F.; Valencia, C.; Pischel, U.; Franco, J.M. Toward UV-Triggered Curing of Solvent-Free Polyurethane Adhesives Based on Castor Oil. ACS Sustain. Chem. Eng. 2021, 9, 11032–11040. [Google Scholar] [CrossRef]
- Li, P.; Chu, Z.; Chen, Y.; Yuan, T.; Yang, Z. One-pot and solvent-free synthesis of castor oil-based polyurethane acrylate oligomers for UV-curable coatings applications. Prog. Org. Coat. 2021, 159, 106398. [Google Scholar] [CrossRef]
- Wang, Z.; Ganewatta, M.S.; Tang, C. Sustainable polymers from biomass: Bridging chemistry with materials and processing. Prog. Polym. Sci. 2020, 101, 101197. [Google Scholar] [CrossRef]
- Constant, E.; King, O.; Weems, A.C. Bioderived 4D Printable Terpene Photopolymers from Limonene and β-Myrcene. Biomacromolecules 2022, 23, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Weems, A.C.; Delle Chiaie, K.R.; Worch, J.C.; Stubbs, C.J.; Dove, A.P. Terpene- and terpenoid-based polymeric resins for stereolithography 3D printing. Polym. Chem. 2019, 10, 5959–5966. [Google Scholar] [CrossRef]
- Zhang, N.; Hou, X.; Cui, X.; Chai, L.; Li, H.; Zhang, H.; Wang, Y.; Deng, T. Amphiphilic catalyst for decomposition of unsaturated polyester resins to valuable chemicals with 100% atom utilization efficiency. J. Clean. Prod. 2021, 296, 126492. [Google Scholar] [CrossRef]
- White, B.T.; Meenakshisundaram, V.; Feller, K.D.; Williams, C.B.; Long, T.E. Vat photopolymerization of unsaturated polyesters utilizing a polymerizable ionic liquid as a non-volatile reactive diluent. Polymer 2021, 223, 123727. [Google Scholar] [CrossRef]
- Cousinet, S.; Ghadban, A.; Fleury, E.; Lortie, F.; Pascault, J.-P.; Portinha, D. Toward replacement of styrene by bio-based methacrylates in unsaturated polyester resins. Eur. Polym. J. 2015, 67, 539–550. [Google Scholar] [CrossRef]
- Lima, M.S.; Costa, C.S.M.F.; Coelho, J.F.J.; Fonseca, A.C.; Serra, A.C. A simple strategy toward the substitution of styrene by sobrerol-based monomers in unsaturated polyester resins. Green Chem. 2018, 20, 4880–4890. [Google Scholar] [CrossRef]
- Pandey, S.; Rajput, B.S.; Chikkali, S.H. Refining plant oils and sugars to platform chemicals, monomers, and polymers. Green Chem. 2021, 23, 4255–4295. [Google Scholar] [CrossRef]
- Lomelí-Rodríguez, M.; Corpas-Martínez, J.R.; Willis, S.; Mulholland, R.; Lopez-Sanchez, J.A. Synthesis and Characterization of Renewable Polyester Coil Coatings from Biomass-Derived Isosorbide, FDCA, 1,5-Pentanediol, Succinic Acid, and 1,3-Propanediol. Polymers 2018, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Llorente, O.; Barquero, A.; Paulis, M.; Leiza, J.R. Challenges to incorporate high contents of bio-based isobornyl methacrylate (IBOMA) into waterborne coatings. Prog. Org. Coat. 2022, 172, 107137. [Google Scholar] [CrossRef]
- Lu, J.; Li, J.; Gao, H.; Zhou, D.; Xu, H.; Cong, Y.; Zhang, W.; Xin, F.; Jiang, M. Recent progress on bio-succinic acid production from lignocellulosic biomass. World J. Microbiol. Biotechnol. 2021, 37, 16. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Jiang, Z.; Qiu, Z. Synthesis, Thermal Behavior, and Mechanical Properties of Fully Biobased Poly(Hexamethylene 2,5-Furandicarboxylate-Co-Sebacate) Copolyesters. Polymers 2022, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhou, C.; Yu, Y.; Li, Y. Biobased copolyesters from renewable resources: Synthesis and crystallization behavior of poly(decamethylene sebacate-co-isosorbide sebacate). RSC Adv. 2015, 5, 42777–42788. [Google Scholar] [CrossRef]
- Lomelí-Rodríguez, M.; Martín-Molina, M.; Jiménez-Pardo, M.; Nasim-Afzal, Z.; Cauët, S.I.; Davies, T.E.; Rivera-Toledo, M.; Lopez-Sanchez, J.A. Synthesis and kinetic modeling of biomass-derived renewable polyesters. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2876–2887. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Vahabi, H.; Rabiee, N.; Rabiee, M.; Bagherzadeh, M.; Saeb, M.R. Green composites in bone tissue engineering. Emergent Mater. 2022, 5, 603–620. [Google Scholar] [CrossRef]
- Robert, T.; Friebel, S. Itaconic acid—A versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016, 18, 2922–2934. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Y.; Dai, J.; Teng, N.; Peng, Y.; Cao, L.; Liu, X. Making organic coatings greener: Renewable resource, solvent-free synthesis, UV curing and repairability. Eur. Polym. J. 2020, 123, 109439. [Google Scholar] [CrossRef]
- Panic, V.V.; Seslija, S.I.; Popovic, I.G.; Spasojevic, V.D.; Popovic, A.R.; Nikolic, V.B.; Spasojevic, P.M. Simple One-Pot Synthesis of Fully Biobased Unsaturated Polyester Resins Based on Itaconic Acid. Biomacromolecules 2017, 18, 3881–3891. [Google Scholar] [CrossRef] [PubMed]
- Maturi, M.; Pulignani, C.; Locatelli, E.; Vetri Buratti, V.; Tortorella, S.; Sambri, L.; Comes Franchini, M. Phosphorescent bio-based resin for digital light processing (DLP) 3D-printing. Green Chem. 2020, 22, 6212–6224. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Teng, N.; Dai, X.; Shen, X.; Wang, S.; Liu, X.; Zhu, J. 2,5-Furandicarboxylic Acid- and Itaconic Acid-Derived Fully Biobased Unsaturated Polyesters and Their Cross-Linked Networks. Ind. Eng. Chem. Res. 2017, 56, 2650–2657. [Google Scholar] [CrossRef]
- Vetri Buratti, V.; Sanz de Leon, A.; Maturi, M.; Sambri, L.; Molina, S.I.; Comes Franchini, M. Itaconic-Acid-Based Sustainable Poly(ester amide) Resin for Stereolithography. Macromolecules 2022, 55, 3087–3095. [Google Scholar] [CrossRef]
- Miao, J.-T.; Peng, S.; Ge, M.; Li, Y.; Zhong, J.; Weng, Z.; Wu, L.; Zheng, L. Three-Dimensional Printing Fully Biobased Heat-Resistant Photoactive Acrylates from Aliphatic Biomass. ACS Sustain. Chem. Eng. 2020, 8, 9415–9424. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Wu, Y.; Han, L.; Zhang, L.; Zhu, J.; Liu, X. Polyesters derived from itaconic acid for the properties and bio-based content enhancement of soybean oil-based thermosets. Green Chem. 2015, 17, 2383–2392. [Google Scholar] [CrossRef]
- Pellis, A.; Hanson, P.A.; Comerford, J.W.; Clark, J.H.; Farmer, T.J. Enzymatic synthesis of unsaturated polyesters: Functionalization and reversibility of the aza-Michael addition of pendants. Polym. Chem. 2019, 10, 843–851. [Google Scholar] [CrossRef]
- Kuenz, A.; Gallenmüller, Y.; Willke, T.; Vorlop, K.-D. Microbial production of itaconic acid: Developing a stable platform for high product concentrations. Appl. Microbiol. Biotechnol. 2012, 96, 1209–1216. [Google Scholar] [CrossRef]
- Saha, B.C.; Kennedy, G.J.; Bowman, M.J.; Qureshi, N.; Nichols, N.N. Itaconic acid production by Aspergillus terreus from glucose up to pilot scale and from corn stover and wheat straw hydrolysates using new manganese tolerant medium. Biocatal. Agric. Biotechnol. 2022, 43, 102418. [Google Scholar] [CrossRef]
- Čuk, N.; Steinbücher, M.; Vidmar, N.; Ocepek, M.; Venturini, P. Fully Bio-Based and Solvent-Free Polyester Polyol for Two-Component Polyurethane Coatings. Coatings 2023, 13, 1779. [Google Scholar] [CrossRef]
- ISO 6271:2015; Clear Liquids—Estimation of Colour by the Platinum-Cobalt Colour Scale. ISO Copyright Office: Geneva, Switzerland, 2015.
- Lee, T.Y.; Roper, T.M.; Jonsson, E.S.; Kudyakov, I.; Viswanathan, K.; Nason, C.; Guymon, C.A.; Hoyle, C.E. The kinetics of vinyl acrylate photopolymerization. Polymer 2003, 44, 2859–2865. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Ragadhita, R.; Fiandini, M. Interpretation of Fourier Transform Infrared Spectra (FTIR): A Practical Approach in the Polymer/Plastic Thermal Decomposition. Indones. J. Sci. Technol. 2022, 8, 113–126. [Google Scholar] [CrossRef]
- González, M.G.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on Epoxy Resins—Identification, Monitoring the Curing Process, Phase Separation and Water Uptake. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophanides, T., Ed.; IntechOpen: London, UK, 2012; ISBN 978-953-51-0537-4. [Google Scholar]
- Sollka, L.; Lienkamp, K. Progress in the Free and Controlled Radical Homo-and Co-Polymerization of Itaconic Acid Derivatives: Toward Functional Polymers with Controlled Molar Mass Distribution and Architecture. Macromol. Rapid Commun. 2021, 42, 2000546. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.; Root, A.; Skrifvars, M.; Ramamoorthy, S.K.; Åkesson, D. Novel Bio-based Branched Unsaturated Polyester Resins for High-Temperature Applications. J. Polym. Environ. 2023, 1–14. [Google Scholar] [CrossRef]
- Cazin, I.; Plevová, K.; Alabiso, W.; Vidović, E.; Schlögl, S. Dual-wavelength vat photopolymerization 3D printing with hybrid acrylate-epoxy resins: Influence of resin composition on microstructure and mechanical properties. Adv. Eng. Mater. 2024; early view. [Google Scholar] [CrossRef]
- Shaukat, U.; Rossegger, E.; Schlögl, S. Thiol–acrylate based vitrimers: From their structure–property relationship to the additive manufacturing of self-healable soft active devices. Polymer 2021, 231, 124110. [Google Scholar] [CrossRef]






| PEA | PEA-BIO-2 | PEA-BIO-3 | |
|---|---|---|---|
| Time of three bottom exposure layers: | 13 s | 20 s | 24 s |
| Exposure time: | 10 s | 15 s | 17 s |
| PEA | PEA-BIO-1 | PEA-BIO-2 | PEA-BIO-3 |
|---|---|---|---|
| cyclohexanedimethanol | cyclohexanedimethanol | isosorbide | isosorbide |
| 1,6-hexanediol | 1,3-propanediol | 1,3-propanediol | 1,3-propanediol |
| adipic acid | sebacic acid | sebacic acid | sebacic acid |
| succinic acid | succinic acid | succinic acid | |
| phtalic acid anhydride | phtalic acid anhydride | phtalic acid anhydride | phtalic acid anhydride |
| acrylic acid | acrylic acid | acrylic acid | itaconic acid |
| 0% bio-based | 40 wt.% bio-based | 60 wt.% bio-based | 95 wt.% bio-based |
| PEA | PEA-BIO-1 | PEA-BIO-2 | PEA-BIO-3 | |
|---|---|---|---|---|
| Acid number (mg KOH/g) | 5.0 | 9.0 | 13.9 | 83.4 |
| Viscosity 23 °C (Pa·s) | 2.31 | 0.27 | 0.96 | 91.78 |
| Color (Gardner) | 3 | 5 | 5.5 | N/A (turbid) |
| Molecular weight (g/mol) | 1543 | 936 | 665 | 1001 |
| Formulation | Tg (°C) | E′ at 23 °C (MPa) | σ (MPa) | ε (%) |
|---|---|---|---|---|
| PEA | 10 | 23.2 | 0.82 ± 0.07 | 6.59 ± 0.62 |
| PEA-BIO-1 | 21 | 103.7 | 1.73 ± 0.23 | 6.89 ± 0.69 |
| PEA-BIO-2 | 19 | 30.7 | 0.61 ± 0.03 | 7.60 ± 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazin, I.; Ocepek, M.; Kecelj, J.; Stražar, A.S.; Schlögl, S. Synthesis of Bio-Based Polyester Resins for Vat Photopolymerization 3D Printing. Materials 2024, 17, 1890. https://doi.org/10.3390/ma17081890
Cazin I, Ocepek M, Kecelj J, Stražar AS, Schlögl S. Synthesis of Bio-Based Polyester Resins for Vat Photopolymerization 3D Printing. Materials. 2024; 17(8):1890. https://doi.org/10.3390/ma17081890
Chicago/Turabian StyleCazin, Ines, Martin Ocepek, Janez Kecelj, Aleš Stanislav Stražar, and Sandra Schlögl. 2024. "Synthesis of Bio-Based Polyester Resins for Vat Photopolymerization 3D Printing" Materials 17, no. 8: 1890. https://doi.org/10.3390/ma17081890
APA StyleCazin, I., Ocepek, M., Kecelj, J., Stražar, A. S., & Schlögl, S. (2024). Synthesis of Bio-Based Polyester Resins for Vat Photopolymerization 3D Printing. Materials, 17(8), 1890. https://doi.org/10.3390/ma17081890