Absorption and Tensility of Bioactive Sutures Prepared for Cell Transplantation
Abstract
:1. Introduction
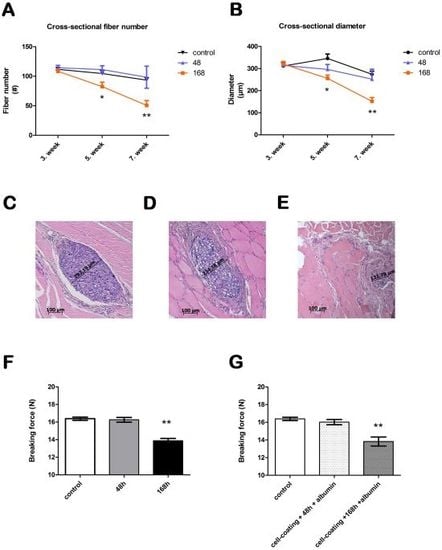
2. Results
2.1. In Vivo Absorption

2.2. Suture Tensility
2.3 Inflammatory Response
3. Discussion

4. Experimental Methods
4.1. Animals
4.2. Suture Preparation
4.3. In Vivo Absorption
4.4. Suture Tensility
4.5. Inflammatory Response
4.6. Statistical Analysis
5. Conclusions
Acknowledgements
References
- Freudenberg, S.; Rewerk, S.; Kaess, M.; Weiss, C.; Dorn-Beinecke, A.; Post, S. Biodegradation of absorbable sutures in body fluids and pH buffers. Eur. Surg. Res. 2004, 36, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, R.; Sonmez, K.; Turkyilmaz, Z.; Bagbanci, B.; Basaklar, A.C.; Kale, N. An in vitro and in vivo evaluation of tensile strength and durability of seven suture materials in various pH and different conditions: An experimental study in rats. Indian J. Surg. 2010, 72, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Korotkova, T.; Smith, R.L. Viability and proliferation of pluripotential cells delivered to tendon repair sites using bioactive sutures—An in vitro study. J. Hand Surg. 2011, 36, 252–258. [Google Scholar] [CrossRef]
- Pascual, I.; de Miguel, G.F.; Gómez Pinedo, U.A.; de Miguel, F.; Arranz, M.G.; García Olmo, D. Adipose derived mesenchymal stem cells in biosutures do not improve healing of experimental colonic anastomoses. Br. J. Surg. 2008, 95, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Georgiev-Hristov, T.; Garcia-Arranz, M.; Garcia-Gomez, I.; Garcia-Cabezas, M.A.; Trebol, J.; Vega-Clemente, L.; Diaz-Agero, P.; Garcia-Olmo, D. Sutures enriched with adipose-derived stem cells decrease the local acute inflammation after tracheal anastomosis in a murine model. Eur. J. Cardiothorac. Surg. 2012, 42, e40–e47. [Google Scholar] [CrossRef] [PubMed]
- Horvathy, D.B.; Vacz, G.; Cselenyak, A.; Weszl, M.; Kiss, L.; Lacza, Z. Albumin-coated bioactive suture for cell transplantation. Surg. Innov. 2012. [Google Scholar] [CrossRef]
- Yao, J.; Woon, C.Y.; Behn, A.; Korotkova, T.; Park, D.Y.; Gajendran, V.; Smith, R.L. The effect of suture coated with mesenchymal stem cells and bioactive substrate on tendon repair strength in a rat model. J. Hand Surg. 2012, 37, 1639–1645. [Google Scholar] [CrossRef]
- Yalcin, L.; Demirci, M.S.; Alp, M.; Akkin, S.M.; Sener, B.; Koebke, J. Biomechanical assessment of suture techniques used for tendon repair. Acta Orthop. Traumatol. Turc. 2011, 45, 453–457. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Horváthy, D.B.; Vácz, G.; Szabó, T.; Renner, K.; Vajda, K.; Sándor, B.; Lacza, Z. Absorption and Tensility of Bioactive Sutures Prepared for Cell Transplantation. Materials 2013, 6, 544-550. https://doi.org/10.3390/ma6020544
Horváthy DB, Vácz G, Szabó T, Renner K, Vajda K, Sándor B, Lacza Z. Absorption and Tensility of Bioactive Sutures Prepared for Cell Transplantation. Materials. 2013; 6(2):544-550. https://doi.org/10.3390/ma6020544
Chicago/Turabian StyleHorváthy, Dénes B., Gabriella Vácz, Tamás Szabó, Károly Renner, Kinga Vajda, Balázs Sándor, and Zsombor Lacza. 2013. "Absorption and Tensility of Bioactive Sutures Prepared for Cell Transplantation" Materials 6, no. 2: 544-550. https://doi.org/10.3390/ma6020544
APA StyleHorváthy, D. B., Vácz, G., Szabó, T., Renner, K., Vajda, K., Sándor, B., & Lacza, Z. (2013). Absorption and Tensility of Bioactive Sutures Prepared for Cell Transplantation. Materials, 6(2), 544-550. https://doi.org/10.3390/ma6020544