Synthesization, Characterization, and in Vitro Evaluation of Cytotoxicity of Biomaterials Based on Halloysite Nanotubes
Abstract
:1. Introduction
2. Experimental Method
2.1. Materials
2.2. Functionalization of HNTs
2.3. Characterization Methods
2.4. Cell Culture
2.5. Cytotoxicity Analysis
| Reaction | Cells | Collagen | HNTs | HNTs–TMPS | HNTS–EOS |
|---|---|---|---|---|---|
| Control | X | – | – | – | – |
| Collagen | X | X | – | – | – |
| HNTs/Collagen | X | X | 0.05% | – | – |
| HNTs–TMPS/Collagen | X | X | – | 0.05% | – |
| HNTs–EOS/Collagen | X | X | – | – | 0.05% |
3. Results and Discussion
3.1. Characterization Results


| Material | O (wt%) | Al (wt%) | Si (wt%) |
|---|---|---|---|
| HNTs | 61.84 | 18.48 | 19.69 |
| HNTs–TMPS | 60.85 | 19.22 | 19.94 |
| HNTs–EOS | 62.57 | 19.92 | 17.51 |

| Sample | Weight Loss in TGA, 200–320 °C (%) | Difference Relative to HNTs (%) |
|---|---|---|
| HNTs | 1.86 | – |
| HNTs–TMPS | 1.94 | 0.08 |
| HNTs–EOS | 2.04 | 0.18 |
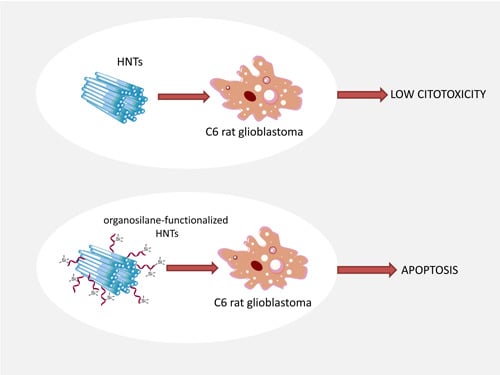
3.2. Cytotoxicity Results

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, Y.; Zhang, B.; Zhang, X.; Wang, J.; Liu, J.; Chen, R. Ammonium removal from aqueous solution by zeolite X synthesized from halloysite mineral. Water Sci. Technol. 2010, 62, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Abdullayev, E.; Lvov, Y. Clay nanotubes for corrosion inhibitor encapsulation: Release control with end stoppers. J. Mater. Chem. 2010, 20, 6681–6687. [Google Scholar] [CrossRef]
- Luo, P.; Zhao, Y.; Zhang, B.; Liu, J.; Yang, Y.; Liu, J. Study on the adsorption of neutral red from aqueous solution onto halloysite nanotubes. Water Res. 2010, 44, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, B.; Zhang, X.; Wang, J.; Liu, J.; Chen, R. Preparation of highly ordered cubic NaA zeolite from halloysite mineral for adsorption of ammonium ions. J. Hazard. Mater. 2010, 178, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Zhang, J.; Zhang, B.; Wang, J.H.; Zhao, Y.F.; Liu, J.D. Preparation and characterization of silane coupling agent modified halloysite for Cr(VI) removal. Ind. Eng. Chem. Res. 2011, 50, 10246–10252. [Google Scholar] [CrossRef]
- Vergaro, V.; Abdullayvev, E.; Lvov, Y.M.; Zeitoun, A. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules 2010, 11, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Abdullayev, E. Functional polymer-clay nanotube composites with sustain release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Shchukin, D.; Price, R.; Sukhorukov, G.; Lvov, Y. Biomimetic synthesis of vaterite in the interior of clay nanotubules. Small 2005, 5, 510–513. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.R.; Hook, J.M.; Antil, S.J.; Kepert, C.J. Functionalization of halloysite clay nanotubes by grafting with γ aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Price, R.; Gaber, B.; Lvov, Y. Release characteristics of tetracycline khellin and NAD from halloysite: A cylindrical mineral for delivery of biologically active agents. J. Microencapsul. 2001, 18, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Kelly, H.; Deasy, P.; Ziaka, E.; Claffey, N. Formulation and preliminary in vivo dog studies of a novel drug delivery system for the treatment of periodontitis. Int. J. Pharm. 2004, 274, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Levis, S.; Deasy, P. Characterisation of halloysite for use as a microtubular drug delivery system. Int. J. Pharm. 2002, 243, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Shchukin, D.; Sukhorukov, G.; Price, R.; Lvov, Y. Halloysite nanotubes as biomimetic nanoreactors. Small 2005, 1, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Kamble, R.; Ghag, M.; Gaikawad, S.; Panda, B.K. Halloysite nanotubes and applications: A review. J. Adv. Sci. Res. 2012, 3, 25–29. [Google Scholar]
- Zhai, R.; Zhang, B.; Liu, L.; Xie, Y.; Zhang, H.; Liu, J. Immobilization of enzyme biocatalyst on natural halloysite nanotubes. Catal. Commun. 2010, 12, 259–263. [Google Scholar] [CrossRef]
- Hughes, A.D.; King, M.R. Use of naturally occurring halloysite nanotubes for enhanced capture of flowing cells. Langmuir 2010, 26, 12155–12164. [Google Scholar] [CrossRef] [PubMed]
- </b>Hughes, A.D.; Mattison, J.; Western, L.; Powderly, J.D.; Greene, B.T.; King, M.R. Microtube device for selectin-mediated capture of viable circulating tumor cells from blood. Clin. Chem. 2012, 58, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.D.; Marshall, J.R.; Keller, E.; Powderly, J.D.; Greene, B.T.; King, M.R. Differential drug responses of circulating tumor cells within patient blood. Cancer Lett. 2014, 352, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Chen, C.S.; Ponmudi, V.; Hughes, A.D.; King, M.R. E-selectin liposomal and nanotube-targeted delivery of doxorubicin to circulating tumor cells. J. Controll. Release 2012, 160, 609–617. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Castellanos, C.A.; King, M.R. Nanostructured surfaces to target and kill circulating tumor cells while repelling leukocytes. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Carli, L.N.; Daitx, T.S.; Soares, G.V.; Crespo, J.S.; Mauler, R.S. The effects of silane coupling agents on the properties of PHBV/halloysite nanocomposites. Appl. Clay Sci. 2014, 87, 311–319. [Google Scholar] [CrossRef]
- Du, M.; Guo, B.; Jia, D. Thermal stability and flame retardant effects of halloysite nanotubes on poly(propylene). Eur. Polym. J. 2006, 42, 1362–1369. [Google Scholar] [CrossRef]
- Liu, M.; Guo, B.; Du, M.; Lei, Y.; Jia, D. Natural inorganic nanotubes reinforced epoxy resin nanocomposites. J. Polym. Res. 2008, 15, 205–212. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Thakur, V.; Mahaling, R.N.; Bhowmick, A.K.; Heinrich, G. Preparation and properties of natural nanocomposites based on natural rubber and naturally occurring halloysite nanotubes. Mater. Des. 2010, 31, 2151–2156. [Google Scholar] [CrossRef]
- Gironès, J.; Méndez, J.A.; Boufi, S.; Vilaseca, F.; Mutjé, P. Effect of silane coupling agents on the properties of pine fibers/polypropylene composites. J. Appl. Polym. Sci. 2007, 103, 3706–3717. [Google Scholar] [CrossRef]
- Shi, Y.F.; Tian, Z.; Zhang, Y.; Shen, H.B.; Jia, N.Q. Functionalized halloysite nanotube-based carrier for intracellular delivery of antisense oligonucleotides. Nanoscale Res. Lett. 2011, 6. [Google Scholar] [CrossRef]
- Sanchez-Fernández, A.; Peña-Parás, L.; Cue-Sampedro, R.; Tamayo, R.; Riojas, P.; Mendoza, A. Synthesization, characterization, and in vitro evaluation of cytotoxicity of biomaterials based on halloysite nanotubes. In Proceedings of the 1st International Electronic Conference on Materials, 26 May–10 June 2014.
- Madani, S.Y.; Mandel, A.; Seifalian, A.M. A concise review of carbon nanotube’s toxicology. Nano Rev. 2013, 4. [Google Scholar] [CrossRef]
- Niles, A.L.; Moravec, R.A.; Riss, T.L. In vitro viability and cytotoxicity testing and same-well multiparametric combinations for high throughput screening. Curr. Chem. Genomics 2009, 3, 31–41. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Fernández, A.; Peña-Parás, L.; Vidaltamayo, R.; Cué-Sampedro, R.; Mendoza-Martínez, A.; Zomosa-Signoret, V.C.; Rivas-Estilla, A.M.; Riojas, P. Synthesization, Characterization, and in Vitro Evaluation of Cytotoxicity of Biomaterials Based on Halloysite Nanotubes. Materials 2014, 7, 7770-7780. https://doi.org/10.3390/ma7127770
Sánchez-Fernández A, Peña-Parás L, Vidaltamayo R, Cué-Sampedro R, Mendoza-Martínez A, Zomosa-Signoret VC, Rivas-Estilla AM, Riojas P. Synthesization, Characterization, and in Vitro Evaluation of Cytotoxicity of Biomaterials Based on Halloysite Nanotubes. Materials. 2014; 7(12):7770-7780. https://doi.org/10.3390/ma7127770
Chicago/Turabian StyleSánchez-Fernández, Antonio, Laura Peña-Parás, Román Vidaltamayo, Rodrigo Cué-Sampedro, Ana Mendoza-Martínez, Viviana C. Zomosa-Signoret, Ana M. Rivas-Estilla, and Paulina Riojas. 2014. "Synthesization, Characterization, and in Vitro Evaluation of Cytotoxicity of Biomaterials Based on Halloysite Nanotubes" Materials 7, no. 12: 7770-7780. https://doi.org/10.3390/ma7127770
APA StyleSánchez-Fernández, A., Peña-Parás, L., Vidaltamayo, R., Cué-Sampedro, R., Mendoza-Martínez, A., Zomosa-Signoret, V. C., Rivas-Estilla, A. M., & Riojas, P. (2014). Synthesization, Characterization, and in Vitro Evaluation of Cytotoxicity of Biomaterials Based on Halloysite Nanotubes. Materials, 7(12), 7770-7780. https://doi.org/10.3390/ma7127770