Silicate Removal in Aluminum Hydroxide Co-Precipitation Process
Abstract
: The removal mechanisms of silicate using an aluminum hydroxide co-precipitation process was investigated and compared with an adsorption process, in order to establish an effective and validated method for silicate removal from wastewater. Adsorption isotherms, XRD and FT-IR analyses showed that silicate uptake occurred by adsorption to boehmite for initial Si/Al molar ratios smaller than two, but by precipitation of poorly crystalline kaolinite for the ratios larger than two, in both co-precipitation and adsorption processes. Silicate was removed by two steps: (i) an initial rapid uptake in a few seconds; and (ii) a slow uptake over several hours in both processes. The uptake rate in the first step was higher in the co-precipitation process than in adsorption process, presumably due to increased silicate adsorption to boehmite and rapid precipitation of kaolinite. These results suggest that silicate removal using aluminum salts could be effectively achieved if the pH adjustment and aluminum concentration are strictly controlled.1. Introduction
Silica is the second most abundant element, comprising 27.7% of the earth’s crust. Its content in natural water is generally between 20 and 60 mg·dm−3, but can be as high as 100 mg·dm−3 in specific natural waters [1]. Although the solution chemistry of silica has been extensively studied, its polymerization or colloidalization in water requires further investigation to understand the mechanism of silica removal from water.
Silica scale, an intermediate product of mono-silicic acid that polymerizes to colloidal silica, clogs water pipes and decreases thermal efficiencies of boilers. Silicate in solution should therefore be removed to a final concentration of less than 10 mg·dm−3. In particular, a decrease in processing efficiency of reverse osmosis (RO) membranes by silica scale is problematic.
RO membrane technology is increasingly being used in chemical engineering separation. For example, after the Great East Japan Earthquake, it was proposed that RO purification could be employed for water extraction from incinerators which hold large quantities of water. This extracted water could be used for both drinking and general use in case of emergency. Silica fouling has been the major unresolved problem in the RO purification process [2,3]. It occurs when dissolved silica exceeds the solubility limit and precipitates on its own or with other compounds. Therefore, prior to using RO membranes for water containing large concentrations of silicate ions, it is necessary to remove the dissolved silica [4,5].
The most common method for removing silicate ions from water is a lime softening process by the addition of slaked lime (calcium hydroxide) [6]. Sodium hydroxide has also been used as an agent for silicate ion removal by precipitation [4]. Other silicate ion removal methods include adsorption or co-precipitation treatments using aluminum or iron salts [5]. Although silicate wastewater treatment using these methods is widely applied, removal mechanisms are unknown.
We investigate the removal mechanisms of silicate co-precipitation with aluminum hydroxide in aqueous systems, in order to establish an effective method of silicate removal from wastewater. Synthetic wastewater containing silicate and aluminum ions was prepared at approximately pH 12, and the pH was then rapidly decreased to pH 9. Because co-precipitation reactions are influenced by solution conditions such as pH and ionic strength, these factors were precisely controlled. Previously, we confirmed that comparison between co-precipitation and simple adsorption was useful for elucidating the removal mechanism for As(V) co-precipitation with ferrihydrite/aluminum hydroxide and F(-I) co-precipitation with aluminum hydroxide [7–11]. Therefore, in this study simple adsorption experiments in which silicate ions were adsorbed to aluminum hydroxide were conducted and compared with co-precipitation experiments. Sorption isotherms were generated and analyzed to identify the mechanism of silicate uptake. The residue was evaluated using X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FT-IR) to investigate the mineralogical form of precipitates and sorption form of silicate in precipitates. Through detailed mechanism elucidation of silicate removal by aluminum salts, we proposed how pH and aluminum ion concentrations should be controlled in order to establish an effective silicate removal method.
2. Materials and Methods
2.1. Standards and Reagents
All chemicals and solutions used in this study were of analytical grade and were purchased from Kanto Chemicals Inc., Tokyo, Japan. The silicate and aluminum solutions were prepared from Na2SiO3·9H2O and Al(NO3)3·9H2O, respectively. For all experiments, the pH and ionic strength were adjusted by the addition of 0.05 M HNO3, KOH and KNO3. Specifically, the pH was fixed at 9 and the ionic strength was fixed at 0.05. All experiments were conducted at 25 °C at least in triplicate and the error was confirmed to be within 1%.
2.2. Co-Precipitation Experiment
Co-precipitation experiments involved the formation of aluminum hydroxide in the presence of silicate ions. Al(NO3)3·9H2O and silicate solutions were initially combined at above pH 12 in 0.5 dm3 of ion-exchanged and deionized (DI) water to maintain both silicate and aluminum as ions. The pH and ionic strength were then adjusted to 9 and 0.05, respectively. The initial silicate concentration was fixed at 0.71 mmol·dm−3 (20 mg·dm−3) or 1.78 mmol·dm−3 (50 mg·dm−3) and the aluminum concentration was varied to adjust the initial Si/Al molar ratio from 0.125 to 10.
The suspension was then agitated using a magnetic stirrer while controlling the pH (accomplished by adding a few drops of KOH) for 1 h. With the exception of the time variation experiments, the reaction time was fixed at 1 h. The suspension was then filtered through a 0.1 μm membrane filter. The filtrates were analyzed by Inductively Coupled Plasma Atomic Emission Spectroscopy: ICP-AES (Seiko Instruments Inc., SPS-7800, Chiba, Japan) to measure the residual concentration of Si and Al.
2.3. Adsorption Experiment
The adsorption experiments were conducted using the same conditions as the co-precipitation experiments. The aluminum hydroxide suspensions and silicate solutions were prepared separately in 0.5 dm3 of deionized and distilled water to obtain twice the target concentration of silicate and aluminum. The pH and ionic strength were adjusted to target levels in both solutions, which were then combined and agitated for 1 h. The pH was adjusted to 9 by the addition of a KOH. After agitation, the suspension was filtered through a 0.1-μm membrane filter, and the filtrate analyzed by ICP-AES to measure the concentration of Si and Al, as well as co-precipitation.
2.4. X-Ray Diffraction (XRD) Analysis
The filter residues of co-precipitation and simple adsorption were analyzed by XRD (RIGAKU, Inc. RINT Ultima III, Tokyo, Japan). For XRD analysis, the initial Al concentration was adjusted to 40 mg·dm−3 and the initial Si concentration was varied according to the Si/Al molar ratio. The filter residues from the co-precipitation and adsorption experiments were freeze-dried at −45 °C and 10 Pa for at least 24 h to avoid crystallization or mineralogical transformation.
Powder XRD patterns were obtained using a copper target (Cu Kα), a crystal graphite monochromator and a scintillation detector. The X-ray source was operated at 40 kV and 30 mA by step-scanning from 2° to 80° 2θ at increments of 0.02° 2θ. A crystal sample holder was used and the diffractograms were not corrected by background diffraction. Powder diffraction files (PDF) from the International Centre for Diffraction Data (ICDD) were used as references using Jade 6.0 software for observation of aluminum hydroxide.
Aluminum hydroxide and aluminum silicate were also analyzed as reference materials. Aluminum hydroxide was prepared from a 40 mg·dm−3 aluminum solution at pH 9. Aluminum silicate was purchased from Kanto Chemicals Inc.
2.5. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
The freeze dried residues analyzed by XRD analysis were also used for FT-IR (JASCO, Inc. FT-IR4200, Tokyo, Japan) analysis. Infrared absorption spectra were recorded on an IR spectrometer using the pressed KBr pellet technique. Approximately 0.6 mg sample and 200 mg KBr were mixed in an agate mortar, and pressed in a 10 mmφ pellet die under a vacuum. The equipment was operated under prescribed conditions with a scanning speed and resolution of 2 mm·s−1 and 4·cm−1, respectively [12].
Aluminum hydroxide and aluminum silicate were also analyzed as reference materials, which were prepared as for XRD analysis.
3. Results and Discussion
3.1. Removal Characteristics
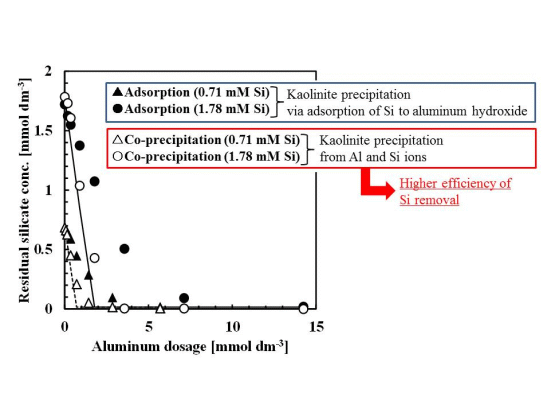
Silicate removal characteristics to aluminum dosage are shown in Figure 1. In these experiments, the pH was 9 and the initial concentration of silicate was 0.71 or 1.78 mmol·dm−3 (20 or 50 mg·dm−3 Si), approximately the same silicate concentration as the wastewater from the incinerator.
The co-precipitation and adsorption experimental results shown in Figure 1 indicated that more silicate was removed using co-precipitation. Solid or dashed lines in Figure 1 show the calculated residual silicate concentration from the thermodynamic solubility constant, K, of kaolinite [Al2Si2O5(OH)4]:
in this calculation, boehmite precipitation was also included because precipitates were characterized as a combination of boehmite and poorly crystalline kaolinite:
the theoretical residual silicate concentration corresponded to the co-precipitation experiments. This observation suggests that the mechanism of silicate uptake in the co-precipitation process was because of kaolinite formation. However, in some plots of 0.71–1.8 mmol·dm−3 aluminum dosage, corresponding to an initial Si/Al molar ratio of 1–2, the theoretical residual silicate was less than the experimental co-precipitation. Under these conditions, kaolinite formation would not have achieved equilibrium within the 1 h reaction time.
3.2. Sorption Isotherm
Figure 2 shows the sorption isotherm obtained from co-precipitation and adsorption experiments at pH 9 after 1 h. In these experiments, the initial silicate concentration was fixed at 0.71 or 1.78 mmol·dm−3 (20 or 50 mg·dm−3 Si) while the Al(III) concentrations ranged from 0.07–14.24 mmol·dm−3 (2–384 mg·dm−3 Al) to obtain an initial Si/Al molar ratio of 0.125, 0.25, 0.5, 1, 2, 5 or 10. Figure 2 shows the initial Si/Al molar ratio only for plots in which the initial Si/Al molar ratio was above 1. In these experiments, sorption density was greater at low aluminum dosages.
Figure 2 indicates that the silicate sorption density for co-precipitation was greater than the adsorption process. In all experiments, sorption isotherms indicated Brunauer-Emmett-Teller (BET) type isotherms, indicating that silicate removal mechanisms in both co-precipitation and adsorption processes involved more than a simple adsorption [7–11]. Silicate sorption density increased substantially when the initial Si/Al molar ratio was >2 in the all experiments, indicating that the mechanism of silicate uptake was three dimensional. This three dimensional uptake may involve adsorption of polymerized silicate in addition to kaolinite precipitation because sorption density exceeded 1 mmol-Si/mmol-Al. We have confirmed previously that multiple complexes such as AlF30 and AlF4− adsorb to aluminum hydroxide, and showed that fluorine sorption density exceeded 1 mmol-F/mmol-Al when the initial F/Al molar ratio was >3 in both co-precipitation and adsorption processes [11].
From Langmuir plots using data of the initial Si/Al molar ratio not greater than two, a linear relationship was obtained and the saturated sorption density was calculated as 0.85 mmol-Si/mmol-Al for the co-precipitation process (solid line in Figure 2) and 0.42 mmol-Si/mmol-Al for the adsorption process (dashed line in Figure 2). The saturated sorption density in co-precipitation was 2-fold greater than observed in the adsorption experiments. In co-precipitation experiments, more silicate could adsorb to aluminum hydroxide because as fresh particles of aluminum hydroxide precipitated, its surface maintained a high capacity for silicate sorption. Additionally, more kaolinite could precipitate during the co-precipitation process because of the aluminum ions present in the solution and kaolinite precipitation did not go through dissolution of aluminum hydroxide, which is necessary for kaolinite formation in the adsorption process.
3.3. XRD Analysis
Figure 3 shows a comparison of XRD spectra of silicate co-precipitated and adsorbed residues as a function of the initial molar ratio of Si/Al. In these experiments, co-precipitation and adsorption were performed at initial Si/Al molar ratios of 0.125, 1, 2, 5 and 10 at pH 9, and the initial aluminum concentration was 1.48 mmol·dm−3 (40 mg·dm−3 Al). The silicate concentration was varied to achieve the target molar ratio. We quantitatively analyzed the Al and Si in the precipitates and confirmed that the Si/Al molar ratio in the precipitates corresponded to the sorption density shown in Figure 2.
Generally, it is difficult to investigate the mineralogical forms of silicate phases in wastewater sludge from XRD patterns because they are poorly crystalline. However, we previously found that surface precipitation could be detected from XRD peak shift in As/Fe or As/Al compounds with different molar ratios [7]. Therefore, XRD spectra were investigated for silicate co-precipitated and adsorbed residues in this study.
As shown in Figures 3 and 4, XRD spectra in co-precipitation and adsorption experiments with the same initial Si/Al molar ratio were similar. This suggests that the mechanism of silicate uptake in co-precipitation and adsorption experiments was very similar, whereas their sorption efficiencies differed.
Included in Figures 3 and 4 are the reference XRD spectra of aluminum hydroxide (Si/Al = 0) and aluminum silicate. The XRD spectrum of aluminum hydroxide had clear broad peaks around 14° 2θ, 28° 2θ, 38° 2θ, 49° 2θ and 65° 2θ, which correspond to poorly crystalline boehmite [13]. Conversely, poorly crystalline aluminum silicate had a clear broad peak around 24.9° 2θ, corresponding to a XRD spectrum of crystalline kaolinite.
When the initial Si/Al molar ratios were smaller than two, silicate co-precipitated or adsorbed residues had a broad and weak peak at 28°, which corresponds to boehmite. This indicates that the main mechanism of silicate uptake was not kaolinite precipitation, but silicate sorption to aluminum hydroxide at an initial Si/Al molar ratio not greater than two in both co-precipitation and adsorption processes. Silicate uptake in both co-precipitation and adsorption processes would make boehmite more amorphous.
The XRD spectra of residues gradually shifted from 28° to 24.9° when the initial Si/Al molar ratio increased from 1 to 10. Because the XRD peak at 24.9° originates from poorly crystalline kaolinite, it is considered that precipitation of amorphous kaolinite increases initial Si/Al molar ratios increase. In addition, the XRD spectrum of residue at Si/Al ratio of 10 was similar to poorly crystalline aluminum silicate, indicating a mechanism of silicate uptake involving the precipitation of aluminum silicate.
It should be noted that the XRD spectrum peak shift from aluminum hydroxide to aluminum silicate was at Si/Al ratio of 2, and that this was consistent with a steep increase in sorption density (Figure 2). Thus for initial Si/Al molar ratios greater than 2, the removal mechanism of silicate was predominantly precipitation of aluminum silicate, such as poorly crystalline kaolinite, which increased silicate sorption density. However, for the ratios smaller than 2, the main removal mechanism of silicate was adsorption to boehmite.
3.4. FT-IR Analysis
Figures 5 and 6 show a comparison of the infrared spectra of silicate co-precipitated and adsorbed residues, as a function of the initial molar ratio of Si/Al. The infrared spectra of aluminum hydroxide obtained at pH 9 (Si/Al = 0) and aluminum silicate are shown as reference data. The FT-IR spectrum peaks are assigned in Table 1 [12–17].
The FT-IR spectra of co-precipitated residues (Figure 5) were almost identical to adsorbed residues (Figure 6). These trends were also observed for XRD analysis (Figures 3 and 4) and indicate a similar mechanism of silicate uptake in co-precipitation and adsorption processes. All the residues showed the OH stretching band at around 3600 cm−1 and a H2O stretching band at 1650 cm−1. In addition, the sharp peak at 1350 cm−1 was attributed to a NO3− stretching band which remained on the surface. A small peak around 1500 cm−1, which was observed at an initial Si/Al molar ratio not greater than 1, originated from CO2 adsorption [18,19]. We confirmed that the particle size of residues decreased with a decrease in the initial Si/Al molar ratio, which suggests that more CO2 would adsorb to residues during Si removal and/or filtration when the initial Si/Al molar ratio was low.
Aluminum hydroxide (Si/Al = 0) had stretching vibration of AlOOH at 1080 cm−1 and a bending vibration and stretching vibration of AlO6 at 600 cm−1 [14], which corresponded with boehmite. Therefore, residues obtained at pH 9 without silicate in this study are most likely boehmite.
Typical absorption bands for kaolinite, e.g., OH vibration bands at around 3650 cm−1, the Si–O–Si absorption bands at 1025 cm−1, the Al–O–Si absorption bands at around 700 cm−1 and the Si–O absorption bands at 432 cm−1 were observed for residues at all initial Si/Al molar ratios. The intensities of these absorption bands gradually increased as the initial Si/Al molar ratio increased, and gradually resembled FT-IR patterns of aluminum silicate. These results suggest that aluminum silicate was poorly crystalline kaolinite and that the mechanism of silicate uptake gradually shifted to precipitation of poorly crystalline kaolinite as the initial Si/Al molar ratio was increased.
3.5. Reaction Rate
Our results suggest that the mechanism of silicate uptake using aluminum hydroxide was influenced by the initial Si/Al molar ratio. Furthermore, the mechanism of silicate uptake in co-precipitation was almost identical to adsorption, whereas removal efficiency and sorption density was higher in co-precipitation. To determine why removal efficiency was so different between co-precipitation and adsorption, time variations of silicate and aluminum concentrations during silicate removal were compared.
Figures 7 and 8 show residual silicate and aluminum concentrations, respectively, over a 24-h period, at pH 9. The initial silicate concentration was 1.78 mmol·dm−3 (50 mg·dm−3 Si) and the initial aluminum concentration was 0.71 or 1.78 mmol·dm−3 to achieve a 1 or 2 initial Si/Al molar ratio.
As shown in Figure 7, silicate removal was achieved in two steps: an initial rapid uptake which occurred within a few seconds, followed by a second, slower silicate uptake taking several hours to equilibrate. When comparing co-precipitation and adsorption, the rapid reaction rate in the first step was different, whereas the slow reaction rate in the second step was almost the same.
This first rapid silicate uptake involved the precipitation of boehmite and kaolinite and silicate adsorption to boehmite. In this step, more silicate could be adsorbed to boehmite in co-precipitation because boehmite was freshly precipitated and had a high capacity for adsorption on its surface. In addition, more kaolinite could precipitate during co-precipitation because aluminum existed as ions in the solution, unlike the adsorption process. Aluminum concentration in co-precipitation was slightly higher than in the adsorption process (Figure 8). At a thermodynamic equilibrium, aluminum concentration equilibration with boehmite was lower than that equilibrated with kaolinite. Therefore, in the adsorption experiments, dissolution of aluminum in boehmite should be rapid to precipitate kaolinite. Conversely, the second slow step would consist of transformation of boehmite to kaolinite. In this step, because boehmite was already precipitated for co-precipitation and adsorption, dissolution of aluminum ions from boehmite and precipitation of kaolinite engaged a longer reaction time of several hours in both processes.
4. Conclusions
Removal mechanisms of silicate using aluminum hydroxide in a co-precipitation process were investigated and compared with an adsorption process. In co-precipitation, higher removal efficiency and sorption density were achieved relative to the adsorption process. The isotherms and results of XRD and FT-IR analysis from co-precipitation experiments were almost identical to those observed from adsorption experiments, which suggested that the main mechanism of silicate uptake was similar for the two methods.
A Langmuir type isotherm was obtained both for co-precipitation and adsorption processes for initial Si/Al molar ratios smaller than 2. Conversely, for the ratios larger than 2, a BET type isotherm was obtained. XRD and FT-IR analysis showed that silicate uptake occurred by adsorption to boehmite for initial Si/Al molar ratios smaller than 2, but by precipitation of poorly crystalline kaolinite for ratios larger than 2, in both co-precipitation and adsorption processes.
Time studies of residual silicate concentrations showed that silicate was removed by two steps: (i) an initial rapid uptake in a few seconds; and (ii) a slow silicate uptake over several hours, in both processes. Only in the first rapid step was the rate of silicate removal higher in co-precipitation relative to the adsorption. The uptake rate in the first step was higher in the co-precipitation process than in adsorption process, presumably due to increased silicate adsorption to boemite and rapid precipitation of kaolinite.
The silicate removal mechanism by aluminum hydroxide suggests that continuous addition of aluminum ions, not aluminum hydroxide particles, should result in highly efficient removal of silicate from wastewater.
Acknowledgments
This research was partially supported by the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Scientific Research (C), 2012-2014 (24561006, Chiharu Tokoro).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Comb, F.L.; Lee, F. Silica Chemistry and Reverse Osmosis. Ultrapure Water 1996, 13, 41–43. [Google Scholar]
- Potts, D.E.; Ahlert, R.C.; Wang, S.S. A critical review of fouling of reverse osmosis membranes. Desalination 1981, 36, 235–264. [Google Scholar]
- Sahachaiyunta, P.; Koo, T.; Sheikholeslami, R. Effect of several inorganic species on silica fouling in RO membranes. Desalination 2002, 144, 373–378. [Google Scholar]
- Sheikholeslami, P.; Al-Mutaz, I.S.; Koo, T.; Young, A. Pretreatment and the effect of cations and anions on prevention of silica fouling. Desalination 2001, 139, 83–95. [Google Scholar]
- Sheikholeslami, R.; Bright, J. Silica and metals removal by pretreatment to prevent fouling of reverse osmosis membranes. Desalination 2002, 143, 255–267. [Google Scholar]
- Sugita, H.; Bamdo, Y.; Nakamura, M. Effects of pH and operational conditions on silica removal from geothermal brine by seeding method using silica gel. Kagaku Kogaku Ronbunshu 1998, 24, 552–557. (in Japanese). [Google Scholar]
- Tokoro, C.; Yatsugi, Y.; Koga, H.; Owada, S. Sorption mechanisms of arsenate during co-precipitation with ferrihydrite in aqueous solution. Environ. Sci. Technol 2010, 44, 638–643. [Google Scholar]
- Tokoro, C.; Haraguchi, D.; Owada, S. Sorption mechanisms of arsenic coprecipitation with ferric or aluminum hydroxides in aqueous solution. Res. Adv. Environ. Sci. Technol 2010, 1, 1–14. [Google Scholar]
- Haraguchi, D.; Tokoro, C.; Oda, Y.; Owada, S. Sorption mechanisms of arsenate in aqueous solution during coprecipitation with aluminum hydroxide. J. Chem. Eng. Jpn 2012, 46, 173–180. [Google Scholar]
- Haraguchi, D.; Oda, Y.; Tokoro, C.; Owada, S. Comparison between aluminum hydroxide coprecipitation and adsorption for removal of dilute As(V) from wastewater. J. MMIJ 2010, 127, 82–87. [Google Scholar]
- Haraguchi, D.; Tokoro, C.; Owada, S. Comparison between aluminum hydroxide coprecipitation and adsorption for removal of fluoride from wastewater. J. MMIJ 2010, 127, 26–31. [Google Scholar]
- Miyawaki, R.; Tomura, S.; Inukai, K.; Shibasaki, Y.; Okazaki, M.; Samejima, S.; Satokawa, S. Formation process of kaolinite from the amorphous mixture of silica and alumina. Clay Sci 1992, 8, 273–284. [Google Scholar]
- Wang, S.G.; Ma, Y.; Shi, Y.J.; Gong, W.X. Defluoridation performance and mechanism of nano-scale aluminum oxide hydroxide in aqueous solution. J. Chem. Technol. Biotechnol 2009, 84, 1043–1050. [Google Scholar]
- Yokoyama, T.; Nishu, K.; Torii, S.; Ikeda, Y. Mullite precursor Part I. Characterization of mullite precursor formed by a reaction of monosilicic acid on aluminum hydroxide. J. Mater. Res 1997, 12, 2111–2116. [Google Scholar]
- Vimont, A.; Lavalley, J.C.; Francke, L.; Demourgues, A.; Tressaud, A.; Daturi, M. Infrared study of the surface properties of HTB-type Al-, Cr-, Fe-hydroxyfluorides. J. Phys. Chem. B 2004, 108, 3246–3255. [Google Scholar]
- Cross, A.D.; Natori, S.; Chihara, G. An Introduction to Practical Infrared Spectroscopy, 3rd ed; Tokyo kagaku doujin: Tokyo, Japan, 1983; pp. 89–119. [Google Scholar]
- Favaro, L.; Boumaza, A.; Roy, P.; Lédion, J.; Sattonnay, G.; Brubach, J.B.; Huntz, A.M.; Tétot, R. Experimental and ab initio infrared study of χ-, κ- and α-aluminas formed from gibbsite. J. Solid State Chem 2010, 183, 901–908. [Google Scholar]
- Arato, T.; Tochigi, K.; Tamamura, T. Characterization for α-Al2O3 and some hydrated aluminas by fourier transform infrared photoacoustic spectroscopy. J. Ceram. Soc. Jpn 1990, 98, 726–731. [Google Scholar]
- AXE, K.; Persson, P. Time-dependent surface speciation of oxalate at the water-boehmite (γ-AlOOH) interface: Implications for dissolution. Geochim. Cosmochim. Acta 2001, 65, 4481–4492. [Google Scholar]








| Band location (cm−1) | Band assignments | Band interaction |
|---|---|---|
| 3650 | −OH | bend |
| 3600 | −OH | stretch |
| 1650 | H2O | stretch |
| 1350 | NO3−, Al−OH | stretch and bond |
| 1105 | Si−O | stretch |
| 1080 | AlOOH | stretch |
| 1025 | Si−O | stretch |
| 700 | Al–O–Si | stretch |
| 600 | AlO6 | stretch and bond |
| 432 | Si–O | stretch |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tokoro, C.; Suzuki, S.; Haraguchi, D.; Izawa, S. Silicate Removal in Aluminum Hydroxide Co-Precipitation Process. Materials 2014, 7, 1084-1096. https://doi.org/10.3390/ma7021084
Tokoro C, Suzuki S, Haraguchi D, Izawa S. Silicate Removal in Aluminum Hydroxide Co-Precipitation Process. Materials. 2014; 7(2):1084-1096. https://doi.org/10.3390/ma7021084
Chicago/Turabian StyleTokoro, Chiharu, Shinya Suzuki, Daisuke Haraguchi, and Sayaka Izawa. 2014. "Silicate Removal in Aluminum Hydroxide Co-Precipitation Process" Materials 7, no. 2: 1084-1096. https://doi.org/10.3390/ma7021084
APA StyleTokoro, C., Suzuki, S., Haraguchi, D., & Izawa, S. (2014). Silicate Removal in Aluminum Hydroxide Co-Precipitation Process. Materials, 7(2), 1084-1096. https://doi.org/10.3390/ma7021084