Film Growth Rates and Activation Energies for Core-Shell Nanoparticles Derived from a CVD Based Aerosol Process
Abstract
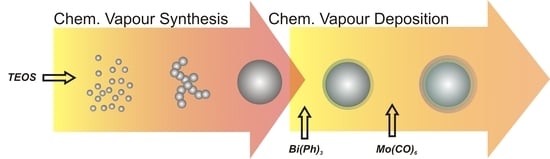
:1. Introduction
2. Results and Discussion

2.1. Molybdenum Oxide Coatings


2.2. Bismuth Oxide Coatings


3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Appendix



Conflicts of Interest
References
- Faust, M.; Enders, M.; Gao, K.; Reichenbach, L.; Muller, T.; Gerlinger, W.; Sachweh, B.; Kasper, G.; Bruns, M.; Bräse, S.; et al. Synthesis of Pt/SiO2 catalyst nanoparticles from a continuous aerosol process using novel cyclo-octadienylplatinum precursors. Chem. Vap. Depos. 2013, 19, 274–283. [Google Scholar] [CrossRef]
- Pratsinis, S.E. Aerosol-based technologies in nanoscale manufacturing: From functional materials to devices through core chemical engineering. AIChE J. 2010, 56, 3028–3035. [Google Scholar] [CrossRef]
- Sigmund, S.; Yu, M.; Meyer, J.; Kasper, G. An aerosol-based process for electrostatic coating of particle surfaces with nanoparticles. Aerosol Sci. Technol. 2013, 48, 142–149. [Google Scholar] [CrossRef]
- Bhakoo, A.; Bond, G.C.; Rees, R.D.; Sauerhammer, B.; Taylor, A.O.; York, I. Supported binary oxide monolayers as selective oxidation catalysts: Bi2Mo3O12/TiO2. Catal. Lett. 1999, 57, 55–60. [Google Scholar] [CrossRef]
- Debecker, D.P.; Schimmoeller, B.; Stoyanova, M.; Poleunis, C.; Bertrand, P.; Rodemerck, U.; Gaigneaux, E.M. Flame-made MoO3/SiO2-Al2O3 metathesis catalysts with highly dispersed and highly active molybdate species. J. Catal. 2011, 277, 154–163. [Google Scholar] [CrossRef]
- Duc, D.T.; Ha, H.N.; Fehrmann, R.; Riisager, A.; Le, M.T. Selective oxidation of propylene to acrolein by silica-supported bismuth molybdate catalysts. Res. Chem. Intermed. 2011, 37, 605–616. [Google Scholar] [CrossRef]
- Ohler, N.; Bell, A.T. Selective oxidation of methane over MoOx/SiO2: Isolation of the kinetics of reactions occurring in the gas phase and on the surfaces of SiO2 and MoOx. J. Catal. 2005, 231, 115–130. [Google Scholar] [CrossRef]
- Greiner, M.T.; Helander, M.G.; Wang, Z.B.; Tang, W.M.; Qiu, J.; Lu, Z.H. A metallic molybdenum suboxide buffer layer for organic electronic devices. Appl. Phys. Lett. 2010, 96, 213302:1–213302:3. [Google Scholar] [CrossRef]
- Cheng, L.; Shao, M.W.; Wang, X.H.; Hu, H.B. Single-crystalline molybdenum trioxide nanoribbons: Photocatalytic, photoconductive, and electrochemical properties. Chem. Eur. J. 2009, 15, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-H.; Chan, C.-H.; Huang, Y.-S.; Tien, L.-C.; Chao, L.-C. The study of optical band edge property of bismuth oxide nanowires α-Bi2O3. Opt. Express 2013, 21, 11965–11972. [Google Scholar] [CrossRef] [PubMed]
- Abdellaoui, A.; Leveque, G.; Donnadieu, A.; Bath, A.; Bouchikhi, B. Iteratively derived optical constants of MoO3 polycrystalline thin films prepared by CVD. Thin Solid Films 1997, 304, 39–44. [Google Scholar] [CrossRef]
- Bandoli, G.; Barreca, D.; Brescacin, E.; Rizzi, G.A.; Tondello, E. Pure and mixed phase Bi2O3 thin films obtained by metal organic chemical vapor deposition. Chem. Vap. Depos. 1996, 2, 238–242. [Google Scholar] [CrossRef]
- Barreca, D.; Rizzi, G.A.; Tondello, E. A chemical vapour deposition route to MoO3-Bi2O3 thin films. Thin Solid Films 1998, 333, 35–40. [Google Scholar] [CrossRef]
- Bedoya, C.; Condorelli, G.G.; Anastasi, G.; Baeri, A.; Scerra, F.; Fragala, I.L.; Lisoni, J.G.; Wouters, D. Mocvd of bismuth oxides: Transport properties and deposition mechanisms of the Bi(C6H5)3 precursor. Chem. Mater. 2004, 16, 3176–3183. [Google Scholar] [CrossRef]
- Diskus, M.; Nilsen, O.; Fjellvag, H.; Diplas, S.; Beato, P.; Harvey, C.; van Schrojenstein Lantman, E.; Weckhuysen, B.M. Combination of characterization techniques for atomic layer deposition MoO3 coatings: From the amorphous to the orthorhombic α-MoO3 crystalline phase. J. Vac. Sci. Technol. A 2012, 30. [Google Scholar] [CrossRef]
- Ivanova, T.; Gesheva, K.A.; Szekeres, A. Structure and optical properties of CVD molybdenum oxide films for electrochromic application. J. Solid State Electrochem. 2002, 7, 21–24. [Google Scholar] [CrossRef]
- Lamouroux, E.; Corrias, M.; Ressier, L.; Kihn, Y.; Serp, P.; Kalck, P. Improving purity and size control of iron- and molybdenum-supported nanoparticles prepared by omcvd from their carbonyl precursors. Chem. Vap. Depos. 2008, 14, 275–278. [Google Scholar] [CrossRef]
- Shi, G.; Franzke, T.; Sánchez, M.D.; Xia, W.; Weis, F.; Seipenbusch, M.; Kasper, G.; Muhler, M. Thin-film β-MoO3 supported on α-Fe2O3 as a shell–core catalyst for the selective oxidation of methanol to formaldehyde. ChemCatChem 2012, 4, 760–765. [Google Scholar] [CrossRef]
- Shi, G.; Franzke, T.; Xia, W.; Sanchez, M.D.; Muhler, M. Highly dispersed MoO3/Al2O3 shell-core composites synthesized by CVD of Mo(CO)6 under atmospheric pressure. Chem. Vap. Depos. 2011, 17, 162–169. [Google Scholar] [CrossRef]
- Reuge, N.; Dexpert-Ghys, J.; Caussat, B. Fluidized-bed mocvd of Bi2O3 thin films from bismuth triphenyl under atmospheric pressure. Chem. Vap. Depos. 2010, 16, 123–126. [Google Scholar] [CrossRef]
- Weis, F.; Seipenbusch, M.; Kasper, G. Coating thickness measurements on gas-borne nanoparticles by combined mobility and aerodynamic spectrometry. J. Nanopart. Res. 2015, 17, 39. [Google Scholar] [CrossRef]
- Loh, W.L.; Jaenicke, S.; Chuah, G.K.; Ang, H.G. Temperature programmed decomposition (TPDE) of [Mo(CO)6] on metal oxide supports: A novel tool to elucidate surface acidity and surface-mediated reactions. Talanta 1998, 45, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Ang, H.-G.; Chan, K.-S.; Chuah, G.-K.; Jaenicke, S.; Neo, S.-K. Thermal reactions of Mo(CO)6 on metal-oxide surfaces. J. Chem. Soc. Dalton Trans. 1995, 1995, 3753–3758. [Google Scholar] [CrossRef]
- Lewis, K.E.; Golden, D.M.; Smith, G.P. Organometallic bond dissociation energies: Laser pyrolysis of Fe(CO)5, Cr(CO)6, Mo(CO)6 and W(CO)6. J. Am. Chem. Soc. 1984, 106, 3905–3912. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Binder, A.; Heel, A.; Kasper, G. Deposition of palladium nanodots of controlled size and density onto surface-modified SiO2 particles by an atmospheric pressure CVS/MOCVD process. Chem. Vap. Depos. 2007, 13, 48–54. [Google Scholar] [CrossRef]
- Steele, W.V. The standard enthalpies of formation of the triphenyl compunds of the Group V elements 2. Triphenylbismuth and the Ph-Bi mean bond-dissociation energy. J. Chem. Thermodyn. 1979, 11, 187–192. [Google Scholar] [CrossRef]
- Weis, F.; Gao, K.; Seipenbusch, M.; Kasper, G. An aerosol-process for the synthesis of nanostructured molybdenum oxide catalysts by integrated chemical vapour synthesis/chemical vapour deposition at atmospheric pressure. J. Nanosci. Nanotechnol. 2011, 11, 8313–8317. [Google Scholar] [CrossRef] [PubMed]
- Weis, F.; Schneider, R.; Seipenbusch, M.; Kasper, G. Synthesis of Bi2O3/SiO2 core-shell nanoparticles by an atmospheric CVS/CVD process and their modification by hydrogen or electron-beam induced reduction. Surf. Coat. Technol. 2013, 230, 93–100. [Google Scholar] [CrossRef]
- Clemente, A.; Balas, F.; Lobera, M.P.; Irusta, S.; Santamaria, J. Fluidized bed generation of stable silica nanoparticle aerosols. Aerosol Sci. Technol. 2013, 47, 867–874. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Wang, S.; Gong, J. The nature of surface acidity and reactivity of MoO3/SiO2 and MoO3/TiO2–SiO2 for transesterification of dimethyl oxalate with phenol: A comparative investigation. Appl. Catal. B Environ. 2007, 77, 125–134. [Google Scholar] [CrossRef]
- Said, A.E.-A.A.; El-Wahab, M.M.A. Surface properties and catalytic behavior of MoO3/SiO2 in esterification of acetic acid with ethanol. J. Chem. Technol. Biotechnol. 2006, 81, 329–335. [Google Scholar] [CrossRef]
- Belhekar, A.A.; Ayyappan, S.; Ramaswamy, A.V. FT-IR studies on the evolution of different phases and their interaction in ferric molybdate-molybdenum trioxide catalysts. J. Chem. Technol. Biotechnol. 1994, 59, 395–402. [Google Scholar] [CrossRef]
- Dhas, N.A.; Gedanken, A. Characterization of sonochemically prepared unsupported and silica-supported nanostructured pentavalent molybdenum oxide. J. Phys. Chem. B 1997, 101, 9495–9503. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weis, F.; Seipenbusch, M.; Kasper, G. Film Growth Rates and Activation Energies for Core-Shell Nanoparticles Derived from a CVD Based Aerosol Process. Materials 2015, 8, 966-976. https://doi.org/10.3390/ma8030966
Weis F, Seipenbusch M, Kasper G. Film Growth Rates and Activation Energies for Core-Shell Nanoparticles Derived from a CVD Based Aerosol Process. Materials. 2015; 8(3):966-976. https://doi.org/10.3390/ma8030966
Chicago/Turabian StyleWeis, Frederik, Martin Seipenbusch, and Gerhard Kasper. 2015. "Film Growth Rates and Activation Energies for Core-Shell Nanoparticles Derived from a CVD Based Aerosol Process" Materials 8, no. 3: 966-976. https://doi.org/10.3390/ma8030966
APA StyleWeis, F., Seipenbusch, M., & Kasper, G. (2015). Film Growth Rates and Activation Energies for Core-Shell Nanoparticles Derived from a CVD Based Aerosol Process. Materials, 8(3), 966-976. https://doi.org/10.3390/ma8030966