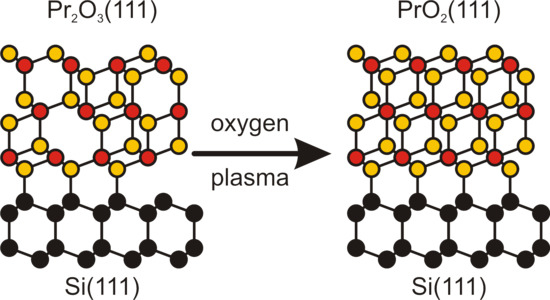
3.1. Surface Characterization
As reported above, the surfaces were characterized by XPS and LEED after initial preparation by annealing at 300 C. The surface diffraction pattern always showed the hexagonal pattern expected for (111) surfaces of films with pseudo-cubic bulk structure. In addition, the pattern exhibited the threefold rotational symmetry of the intensities of the higher order diffraction peaks expected due to the ABC stacking of the bulk material.
Typical Pr 3d and Pr 4d photoemission spectra (blue lines) obtained after the initial annealing preparation are presented in
Figure 1a,b, respectively. The spectra agree well with former photoemission spectra of Pr
O
which were calculated and analyzed in detail by Kotani and Ogasawara [
14,
15]. All cations in Pr
O
have Pr
valence state. Thus, the ground state exhibits a 4f
configuration, which leads in the final state of Pr3d photoemission spectra to two final states in each 3d
and 3d
component. The emission peaks in
Figure 1a denoted by
u and
v are attributed to the bonding 4f
and anti-bonding 4f
configuration, respectively, which are strongly affected by the covalency hybridization induced by the core hole potential. Here
denotes a hole in the O2p valence band. The spin orbit splitting is 20.6 eV. Beyond 970 eV the Auger O KLL emission can be seen for the investigated emission range.
Figure 1.
Pr 3d (a) and Pr 4d (b) photoemission spectra from praseodymia films before (blue lines) and after plasma treatment (red lines). (a) Bonding and anti-bonding states of Pr (PrO stoichiometry) are denoted by u and v, respectively, while these states are denoted by x and y for Pr (PrO stoichiometry). In addition, z denotes the pure main emission here; (b) Pr 4d photoelectrons due to higher binding energies are observed after the transformation from PrO to PrO.
Figure 1.
Pr 3d (a) and Pr 4d (b) photoemission spectra from praseodymia films before (blue lines) and after plasma treatment (red lines). (a) Bonding and anti-bonding states of Pr (PrO stoichiometry) are denoted by u and v, respectively, while these states are denoted by x and y for Pr (PrO stoichiometry). In addition, z denotes the pure main emission here; (b) Pr 4d photoelectrons due to higher binding energies are observed after the transformation from PrO to PrO.
The Pr 4d spectrum of the Pr
O
films presented in
Figure 1a is governed by multiplet coupling effects, due to exchange interaction between 4d and 4f states. The spin-orbit-coupling in the 4d states has a minor impact than the exchange interaction between 4d and 4f states, so the signal is not separated into 4d
and 4d
[
15].
As demonstrated by
Figure 1a,b, the Pr 3d and Pr 4d photoelectron emission spectra (red lines) change drastically if the praseodymia films are oxidized by plasma treatment. Here, the ground state consists of a mixing between 4f
and 4f
configurations which leads in the final state of Pr 3d photoemission spectra to three final states in each Pr 3d
and Pr 3d
emission line. The peaks denoted by
x and
y correspond to the the bonding and antibonding final states of the strong mixed 4f
and 4f
configuration while the peaks denoted by
z are attributed to the pure 4f
main line [
16]. The spin-orbit splitting is 20.6 eV, too.
Comparing the spectra obtained from the Pr
O
and PrO
films obtained before and after plasma treatment, respectively, one can clearly see differences in the spectral shape of the Pr 3d photoemission signal due to the oxidation in oxygen plasma (
Figure 1a). This is a clear evidence that the surface near region of the plasma treated films are completely oxidized and exhibit the PrO
stoichiometry.
The Pr 4d spectrum of the plasma treated films presented in
Figure 1b range from 106 eV to 135 eV binding energy. Compared to the Pr 4d spectrum obtained from Pr
O
, there is a shift of the spectral shape from lower to higher binding energies for plasma oxidized films.
We like to emphasize that we observe very similar photoemission spectra for all plasma treatments used in our studies and for all investigated thicknesses of the praseodymia film. All spectra agree well with the spectra reported for tetravalent Pr 3d spectra in literature [
10,
17]. Hence, oxygen plasma treatment leads to the formation of PrO
in surface near regions.
3.2. Film Thickness
Having performed the initial cleaning procedure, PrO films of different thickness have been exposed to oxygen plasma at 26 Pa O for 15 min using an O flux of 40 sccm.
Figure 2a compares SR-XRR data recorded before and after this treatment for Pr
O
films with thickness ranging from 6 nm to 18 nm. All reflectivity curves show Kiessig fringes which have been used to determine the film thickness
a posteriori with an accuracy better than 0.3 nm. After plasma treatment, the strength of the intensity oscillations obviously decreases for the 9 nm and the 18 nm sample while the oscillation is clearer for the 6 nm sample. This can be attributed to changes of surface roughness caused by the plasma oxidation process. The roughness of the thicker films has increased while the 6 nm film becomes smoother.
Figure 2.
SR-XRR (a) and SR-XRD ((00L) crystal truncation rod) (b) data obtained from praseodymia films of different thickness: 6 nm (green line), 9 nm (blue line) and 18 nm (red line). The lower curves are obtained from the PDA treated samples while the upper curves are obtained after additional oxygen plasma treatment (26 Pa O, 40 sccm flux, 15 min). The reference films without plasma treatment show higher oxidation states due to oxidation under ambient conditions. After plasma treatment the 9 nm and 18 nm films are complete oxidized while the 6 nm film is only partly oxidized.
Figure 2.
SR-XRR (a) and SR-XRD ((00L) crystal truncation rod) (b) data obtained from praseodymia films of different thickness: 6 nm (green line), 9 nm (blue line) and 18 nm (red line). The lower curves are obtained from the PDA treated samples while the upper curves are obtained after additional oxygen plasma treatment (26 Pa O, 40 sccm flux, 15 min). The reference films without plasma treatment show higher oxidation states due to oxidation under ambient conditions. After plasma treatment the 9 nm and 18 nm films are complete oxidized while the 6 nm film is only partly oxidized.
For a complete analysis of the SR-XRR data, we used the Parratt algorithm [
18] combined with the model of Nevot and Croce to implement the surface roughness [
19]. The fitting procedure revealed that the rms-roughness of the films is roughly 1 Å before plasma treatment. Changes of the rms-roughness after plasma treatment is very small (in the range of few 0.1 Å).
In addition, the index of refraction of the film can be obtained from the fitting procedure with an accuracy better than 2%. This result is related to the electron density of the studied praseodymia films. In the following, we assume the pseudo cubic lattice constant for the different praseodymia phases and consider their stoichiometry to calculate their electron densities. Before oxidation the praseodymia films showed an electron density of 1.80 Å. This result is very close to the calculated value of 1.78 Å expected for PrO phase which is stable under ambient conditions. Therefore, we conclude that the original PrO films had transformed to PrO before they were inserted in the plasma chamber. However, it has to be emphasized that the films revealed PrO stoichiometry after initial annealing procedure performed in the preparation chamber before plasma oxidation (cf. above).
After oxidation the electron density of the praseodymia films increased to 1.90 Å. If compared to the calculated value of 1.92 Å expected for PrO, this results demonstrates the effectiveness of the plasma oxidation procedure. Within the experimental error the films are completely oxidized. This result agrees well with our XPS studies presented above. In addition, one has to conclude that PrO films are much more stable under ambient conditions than PrO films are.
Finally, we like to point to the beating effect observed in the SR-XRR data. This effect has to be attributed to the existence of an additional very thin interlayer between praseodymia film and Si(111) substrate. Our analysis shows that the thickness of this interlayers is 1.4 (±0.4) nm and that the electron density of the interlayers is 1.2 (±0.1) Å
. Therefore, we conclude that the interlayers are formed by silicate due to interface reaction before. This effect is well known in literature where it is also reported that these films are amorphous [
8,
20].
These praseodymia films were investigated by SR-XRD, too. The results are presented in
Figure 2b after scanning the
Crystal Truncation Rod (CTR) close to the second Bragg condition
L = 2. Here, we use the surface notation to describe the diffraction geometry. Thus, the vertical component
of the scattering vector is related to the distance
of Si(111) bulk layers and
L denotes the scaled vertical component
L = 2
.
Figure 2b shows that, before plasma treatment, the Bragg peaks of the praseodymia films have smaller
L values than the Si(111) Bragg peak. Thus the praseodymia layer distance
c is larger than the Si(111) layer distance
. In addition, the expected layer distances for the common praseodymia phases Pr
O
, Pr
O
and PrO
are denoted in
Figure 2b. Here, we present ranges of possible Bragg peak positions for the cases ranging from pseudomorphic elastically distorted films to completely relaxed films having bulk value layer distances. Furthermore, we performed a detailed analysis of the diffraction data based on the kinematic approximation (black lines in
Figure 2b) which will be discussed below.
The positions of the praseodymia Bragg peaks are larger than expected for Pr
O
and shifted to the values expected for higher oxygen content of the praseodymia films. Thus the praseodymia films are oxidized under ambient conditions as concluded before from the SR-XRR data. This effect is well known for praseodymia powder where it is reported that Pr
O
is the stable phase under ambient conditions [
21,
22].
Obviously, with increasing film thickness, the position of the praseodymia Bragg peaks shift further to higher values pointing to a decreasing layer distance. This effect can be explained by an increasing oxidation state of the praseodymia since the average size of cations decreases for higher oxidation states and the bulk lattice constant consequently shrinks (
cf. also the noted positions of the different praseodymia phases in
Figure 2b).
After plasma oxidation the positions of the Bragg peak are shifted to higher values for all investigated praseodymia films. Thus, all films have successfully been oxidized. The oxidation state of the thinnest 6 nm film, however, is far from being PrO. Instead it seems, that the film finally received PrO stoichiometry although our XPS studies show the complete oxidation of the surface near region. Therefore, it seems that the interface stabilizes smaller degrees of oxidation.
On the other hand, the thicker 9 nm and 18 nm films clearly show Bragg peaks which can be attributed to PrO
. A detailed kinematic analysis of the diffraction data showed that the layer distance is
c = 307 (±1) pm for both thicker films. This value is smaller than the layer distance
c = 310 pm expected from bulk data for relaxed PrO
films [
21,
22] and can also not be explained by elastic distortion of the film due to lattice matching at the interface. In the latter case, one would expect a layer distance of
c = 309 pm. Thus the film seems to be “over oxidized”. However, the obtained layer distance agree well with previously published data for PrO
films which have been oxidized by oxygen plasma [
11].
Furthermore, it has to be emphasized that all diffraction scans except the plasma oxidized 18 nm film clearly show Laue fringes. These fringes are caused by single crystalline films with well defined film thickness and low interface and surface roughness. Generally, the intensity of the Laue fringes is smaller after plasma oxidation. Thus, the oxidation process roughens the praseodymia films. This result agrees well with our SR-XRR result (see above).
In addition, the thickness of the crystalline part of the films studied here is slightly smaller than the film thickness obtained from SR-XRR. This effect can be attributed to the non-crystalline interface layer reported before from our SR-XRR results. Furthermore, the kinematic diffraction analysis shows also that there exist crystalline interlayers which are less oxidized than the oxide film on top. This result agrees well with Gevers
et al., who reported residual interlayers with PrO
stoichiometry [
11].
Finally, we like to emphasize that there seems to be some residual diffraction intensity close to the Bragg position of the initial Pr
O
film for the plasma oxidized 9 nm film. Thus, this film seems not to be completely transformed to PrO
. This result also agrees well with the results obtained by Gevers
et al., who found some lateraly separated phases in plasma oxidized praseodymia films [
11]. Therefore, we performed some further studies on this film.
3.3. Exposure Time
We studied the effect of exposure time in a second series of experiments performed on praseodymia films of 9 nm thickness. Here, we kept the flux of 40 sccm O as well as the O pressure of 26 Pa constant.
In
Figure 3a we present the SR-XRR data recorded after treating the praseodymia films from 5 min to 15 min. No clear changes of the SR-XRR data can be seen increasing the time of treatment. A detailed analysis fitting the data (black curves) shows that the electron density slightly increases already after the 5 min treatment. Further treatment does not cause significant improvements. Time dependent effects after the first treatment can also not be observed concerning the rms-roughness of the film as well as thickness, electron density and interface roughness of the amorphous interlayer.
Figure 3.
SR-XRR (a) and SR-XRD (b) data obtained from 9 nm praseodymia films after different plasma treatment times (5–15 min) keeping other parameters (26 Pa O, 40 sccm flux) constant. Complete oxidation is already obtained after the first 5 min oxidation step.
Figure 3.
SR-XRR (a) and SR-XRD (b) data obtained from 9 nm praseodymia films after different plasma treatment times (5–15 min) keeping other parameters (26 Pa O, 40 sccm flux) constant. Complete oxidation is already obtained after the first 5 min oxidation step.
Similar conclusions can also be drawn from the SR-XRD experiments on these film presented in
Figure 3b. The position of the Bragg peak shifts clearly to a higher
L value after the first treatment of 5 min. From the complete kinematic analysis we obtain that the layer distance of the praseodymia film is
c = 306 (±1) pm pointing to a complete oxidation of the film which assumes PrO
stoichiometry and structure. However, parts of the film seem still to have less oxidized stoichiometries. Furthermore, a less oxidized crystalline interface layer is detected by kinematic diffraction analysis of the SR-XRD data.
All CTRs of the plasma treated films have Laue oscillations from which we conclude that the films show homogeneous film thickness. The thickness of the crystalline parts of the film do not change with increasing treatment time.