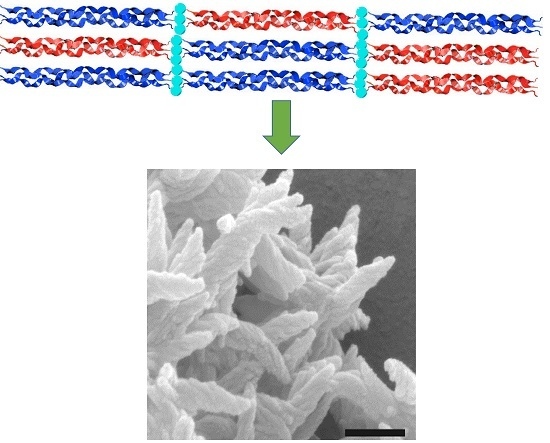
Metal-Promoted Assembly of Two Collagen Mimetic Peptides into a Biofunctional “Spiraled Horn” Scaffold
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Peptides
2.3. Metal-Ion-Promoted Assembly
2.4. Scanning Electron Microscopy (SEM)
2.5. Transmission Electron Microscopy (TEM)
2.6. Preparation and Visualization of Rho-HIS-Labeled “Spiraled Horn” Scaffolds
2.7. Cell-Binding Study
3. Results and Discussion
3.1. SEM Characterization of Assemblies
3.2. TEM Characterization of Assemblies
3.3. The Effect of Strand Exchange within the Triple Helices
3.4. Metal Concentration Dependency on Assembly Morphology
3.5. Incubation Time Dependency on Assembly Morphology
3.6. Decoration by a Histidine (His)-Tagged Fluorophore
3.7. Interaction between “Spiraled Horn” Scaffolds and HeLa Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ricard-Blum, S.; Ruggiero, F. The collagen superfamily: From the extracellular matrix to the cell membrane. Pathol. Biol. 2005, 53, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Bansal, M. Collagen structure: The Madras triple helix and the current scenario. IUBMB Life 2005, 57, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Raines, R.T. Interstrand dipole-dipole interactions can stabilize the collagen triple helix. J. Biol. Chem. 2011, 286, 22905–22912. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Hori, A.; Sugaya, S.; Yajima, Y.; Utoh, R.; Yamato, M.; Seki, M. Cell-sized condensed collagen microparticles for preparing microengineered composite spheroids of primary hepatocytes. Lab Chip 2015, 15, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Phan, F.; Li, Y. Collagen microsphere serving as a cell carrier supports oligodendrocyte progenitor cell growth and differentiation for neurite myelination in vitro. Stem Cell Res. Ther. 2013, 4, 109. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Kumasaka, N.; Kawashima, T.; Kaji, H.; Nishizawa, M.; Abe, T. Preparation and characterization of collagen microspheres for sustained release of VEGF. J. Mater. Sci. Mater. Med. 2010, 21, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, S.; Yamada, M.; Hori, A.; Seki, M. Microfluidic production of single micrometer-sized hydrogel beads utilizing droplet dissolution in a polar solvent. Biomicrofluidics 2013, 7, 54120. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Yamada, M.; Suzuki, Y.; Taniguchi, T.; Seki, M. One-step synthesis of spherical/nonspherical polymeric microparticles using non-equilibrium microfluidic droplets. RSC Adv. 2014, 4, 13557–13564. [Google Scholar] [CrossRef]
- Paramonov, S.E.; Gauba, V.; Hartgerink, J.D. Synthesis of Collagen-like Peptide Polymers by Native Chemical Ligation. Macromolecules 2005, 38, 7555–7561. [Google Scholar] [CrossRef]
- Chen, C.C.; Hsu, W.; Kao, T.C.; Horng, J.C. Self-assembly of short collagen-related peptides into fibrils via cation-pi interactions. Biochemistry 2011, 50, 2381–2383. [Google Scholar] [CrossRef] [PubMed]
- Krishna, O.D.; Kiick, K.L. Supramolecular Assembly of Electrostatically Stabilized, Hydroxyproline-Lacking Collagen-Mimetic Peptides. Biomacromolecules 2009, 10, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Kotch, F.W.; Raines, R.T. Self-assembly of synthetic collagen triple helices. Proc. Natl. Acad. Sci. USA 2006, 103, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Rele, S.; Song, Y.; Apkarian, R.P.; Qu, Z.; Conticello, V.P.; Chaikof, E.L. D-Periodic Collagen-Mimetic Microfibers. J. Am. Chem. Soc. 2007, 129, 14780–14787. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Xu, C.; Liu, Y.; Liu, Z.; Wall, J.S.; Zuo, X.; Lian, T.; Salaita, K.; Ni, C.; Pochan, D.; et al. Structurally defined nanoscale sheets from self-assembly of collagen-mimetic peptides. J. Am. Chem. Soc. 2014, 136, 4300–4308. [Google Scholar] [CrossRef] [PubMed]
- Cejas, M.A.; Kinney, W.A.; Chen, C.; Vinter, J.G.; Almond, H.R., Jr.; Balss, K.M.; Maryanoff, C.A.; Schmidt, U.; Breslav, M.; Mahan, A.; et al. Thrombogenic collagen-mimetic peptides: Self-assembly of triple helix-based fibrils driven by hydrophobic interactions. Proc. Natl. Acad. Sci. USA 2008, 105, 8513–8518. [Google Scholar] [CrossRef] [PubMed]
- Kar, K.; Ibrar, S.; Nanda, V.; Getz, T.M.; Kunapuli, S.P.; Brodsky, B. Aromatic interactions promote self-association of collagen triple-helical peptides to higher-order structures. Biochemistry 2009, 48, 7959–7968. [Google Scholar] [CrossRef] [PubMed]
- Przybyla, D.E.; Chmielewski, J. Higher-order assembly of collagen peptides into nano- and microscale materials. Biochemistry 2010, 49, 4411–4419. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; He, L.; Theato, P.; Kiick, K.L. Thermoresponsive Self-Assembly of Nanostructures from a Collagen-Like Peptide-Containing Diblock Copolymer. Macromol. Biosci. 2015, 15, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Tanrikulu, I.C.; Forticaux, A.; Jin, S.; Raines, R.T. Peptide tessellation yields micrometre-scale collagen triple helices. Nat. Chem. 2016. [Google Scholar] [CrossRef]
- Przybyla, D.E.; Chmielewski, J. Metal-Triggered Radial Self-Assembly of Collagen Peptide Fibers. J. Am. Chem. Soc. 2008, 130, 12610–12611. [Google Scholar] [CrossRef] [PubMed]
- Przybyla, D.E.; Chmielewski, J. Metal-Triggered Collagen Peptide Disk Formation. J. Am. Chem. Soc. 2010, 132, 7866–7867. [Google Scholar] [CrossRef] [PubMed]
- Przybyla, D.E.; Rubert Perez, C.M.; Gleaton, J.; Nandwana, V.; Chmielewski, J. Hierarchical assembly of collagen peptide triple helices into curved disks and metal ion-promoted hollow spheres. J. Am. Chem. Soc. 2013, 135, 3418–3422. [Google Scholar] [CrossRef] [PubMed]
- Gleaton, J.; Chmielewski, J. Thermally Controlled Collagen Peptide Cages for Biopolymer Delivery. ACS Biomater. Sci. Eng. 2015, 1, 1002–1008. [Google Scholar] [CrossRef]
- Pires, M.M.; Chmielewski, J. Self-assembly of Collagen Peptides into Microflorettes via Metal Coordination. J. Am. Chem. Soc. 2009, 131, 2706–2712. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.M.; Przybyla, D.E.; Rubert Perez, C.M.; Chmielewski, J. Metal-mediated tandem coassembly of collagen peptides into banded microstructures. J. Am. Chem. Soc. 2011, 133, 14469–14471. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.M.; Przybyla, D.E.; Chmielewski, J. A metal-collagen peptide framework for three-dimensional cell culture. Angew. Chem. Int. Ed. Engl. 2009, 48, 7813–7817. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gordillo, V.; Chmielewski, J. Mimicking the extracellular matrix with functionalized, metal-assembled collagen peptide scaffolds. Biomaterials 2014, 35, 7363–7373. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Colescott, R.L.; Bossinger, C.D.; Cook, P.I. Color Test for Detection of Free Terminal Amino Groups in the Solid-Phase Synthesis of Peptides. Anal. Biochem. 1970, 34, 595–598. [Google Scholar] [CrossRef]
- Christensen, T. A Qualitative Test for Monitoring Coupling Completeness in Solid Peptide Synthesis Using Chloranil. Acta Chem. Scand. B 1979, 33, 763–766. [Google Scholar] [CrossRef]
- Quan, B.D.; Sone, E.D. Structural changes in collagen fibrils across a mineralized interface revealed by cryo-TEM. Bone 2015, 77, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Tay, F.R.; Ohno, H.; Sano, H.; Kaga, M.; Yiu, C.; Kumagai, H.; Kudou, Y.; Kubota, M.; Oguchi, H. SEM and TEM Analysis of Water Degradation of Human Dentinal Collagen. J. Biomed. Mater. Res. B 2003, 66, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.M.; Ernenwein, D.; Chmielewski, J. Selective decoration and release of His-tagged proteins from metal-assembled collagen peptide microflorettes. Biomacromolecules 2011, 12, 2429–2433. [Google Scholar] [CrossRef] [PubMed]
- Scherer, W.F.; Syverton, J.T.; Gey, G.O. Studies on the Propagation In Vitro of Poliomyelitis Viruses IV. Viral Multiplicatin in a Stable Strain of Human Malignant Epithelial Cells (Strain HeLa) Derived from an Epidermoid Carcinoma of the Cervix. J. Exp. Med. 1953, 97, 695–710. [Google Scholar]
- Rahbari, R.; Sheahan, T.; Modes, V.; Collier, P.; Macfarlane, C.; Badge, R.M. A novel L1 retrotransposon marker for HeLa cell line identification. Biotechniques 2009, 46, 277–284. [Google Scholar] [PubMed]








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strauss, K.; Chmielewski, J. Metal-Promoted Assembly of Two Collagen Mimetic Peptides into a Biofunctional “Spiraled Horn” Scaffold. Materials 2016, 9, 838. https://doi.org/10.3390/ma9100838
Strauss K, Chmielewski J. Metal-Promoted Assembly of Two Collagen Mimetic Peptides into a Biofunctional “Spiraled Horn” Scaffold. Materials. 2016; 9(10):838. https://doi.org/10.3390/ma9100838
Chicago/Turabian StyleStrauss, Kevin, and Jean Chmielewski. 2016. "Metal-Promoted Assembly of Two Collagen Mimetic Peptides into a Biofunctional “Spiraled Horn” Scaffold" Materials 9, no. 10: 838. https://doi.org/10.3390/ma9100838
APA StyleStrauss, K., & Chmielewski, J. (2016). Metal-Promoted Assembly of Two Collagen Mimetic Peptides into a Biofunctional “Spiraled Horn” Scaffold. Materials, 9(10), 838. https://doi.org/10.3390/ma9100838