Newly Developed Techniques on Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus on Poly(Lactic Acid)
Abstract
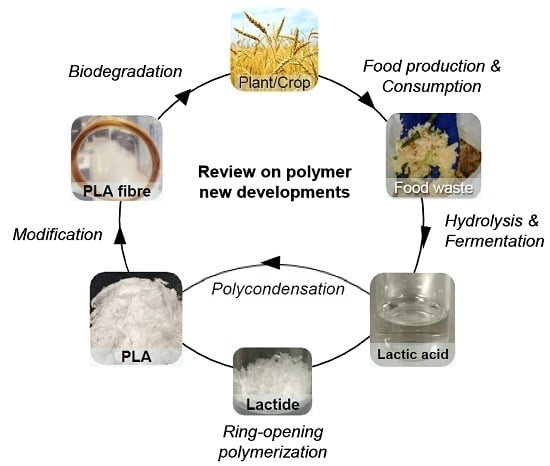
:1. Introduction
2. PLA Synthesis
3. Polymer Synthesis by Polycondensation
Recent Development in PLA Synthesis by Polycondensation
4. Polymer Synthesis by Ring-Opening Polymerization
4.1. Recent Development in PLA Synthesis Method through Ring Opening Polymerization
4.1.1. Lactide Synthesis
4.1.2. Polymerization of Lactide
5. Newly Developed PLA Modification Techniques
5.1. Bulk Modification
5.2. Surface Modification
6. Comparison and New Trends of Poly(Lactic Acid) Synthesis and Modification Development
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AP | Azeotropic polycondensation |
| BDPs | Biodegradable plastics |
| DP | Direct polycondensation |
| Mw | Molecular weight |
| KT | Kilo tonnes |
| PCL | Poly(e-caorolactone) |
| PEG | Poly(ethylene glycol) |
| PGL | polyglycerine |
| PLGA | Poly(lactic acid-co-glycolic acid) |
| PLA | Poly(lactic acid) |
| ROP | Ring-opening polymerization |
| SIMes | 1,3-Bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene |
| SSP | Solid state polycondensation |
| T | Temperature |
| TBD | 1,5,7-Triazabicyclo[4.4.0]dec-5-ene |
| P | Pressure |
References
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, K.; Ohtaki, A.; Takano, H. Biodegradable plastic reduces ammonia emission during composting. Polym. Degrad. Stab. 2000, 70, 185–188. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S. Synthesis of poly(lactic acid): A review. J. Macromol. Sci. Polym. Rev. 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, V. New emerging trends in synthetic biodegradable polymers–polylactide: A critique. Eur. Polym. J. 2007, 43, 4053–4074. [Google Scholar] [CrossRef]
- Carothers, W.H.; Dorough, G.; Natta, F.V. Studies of polymerization and ring formation. X. The reversible polymerization of six-membered cyclic esters. J. Am. Chem. Soc. 1932, 54, 761–772. [Google Scholar] [CrossRef]
- Thomas, C.M. Stereocontrolled ring-opening polymerization of cyclic esters: Synthesis of new polyester microstructures. Chem. Soc. Rev. 2010, 39, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Kimura, Y. An efficient solid-state polycondensation method for synthesizing stereocomplexed poly(lactic acid)s with high molecular weight. J. Polym. Sci. A Polym. Chem. 2008, 46, 3714–3722. [Google Scholar] [CrossRef]
- Achmad, F.; Yamane, K.; Quan, S.; Kokugan, T. Synthesis of polylactic acid by direct polycondensation under vacuum without catalysts, solvents and initiators. Chem. Eng. J. 2009, 151, 342–350. [Google Scholar] [CrossRef]
- Nagahata, R.; Sano, D.; Suzuki, H.; Takeuchi, K. Microwave-assisted single-step synthesis of poly(lactic acid) by direct polycondensation of lactic acid. Macromol. Rapid Commun. 2007, 28, 437–442. [Google Scholar] [CrossRef]
- Kim, K.W.; Woo, S.I. Synthesis of high-molecular-weight poly (l-lactic acid) by direct polycondensation. Macromol. Chem. Phys. 2002, 203, 2245–2250. [Google Scholar] [CrossRef]
- Fukushima, K.; Furuhashi, Y.; Sogo, K.; Miura, S.; Kimura, Y. Stereoblock poly (lactic acid): Synthesis via solid-state polycondensation of a stereocomplexed mixture of poly (l-lactic acid) and poly (d-lactic acid). Macromol. Biosci. 2005, 5, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Nuyken, O.; Pask, S.D. Ring-opening polymerization-an introductory review. Polymers 2013, 5, 361–403. [Google Scholar] [CrossRef]
- Stridsberg, K.M.; Ryner, M.; Albertsson, A.C. Controlled ring-opening polymerization: Polymers with designed macromolecular architecture. In Degradable Aliphatic Polyesters, 1st ed.; Albertsson, A.C., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; Volume 157, pp. 41–65. [Google Scholar]
- Zhang, J.; Krishnamachari, P.; Lou, J.; Shahbazi, A. Synthesis of poly(L(+) lactic acid) by polycondensation method in solution. In Proceedings of the 2007 National Conference on Environmental Science and Technology, Greensboro, NC, USA, 12–14 September 2007; Uzochukwu, G., Schimmel, K., Chang, S.Y., Kabadi, V., Luster-Teasley, S., Reddy, G., Nzewi, E., Eds.; Springer: New York, NY, USA, 2009; pp. 3–8. [Google Scholar]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Avérous, L. Polylactic acid: Synthesis, properties and applications. In Monomers, Polymers and Composites from Renewable Resources, 1st ed.; Mohamed, N.B., Alessandro, G., Eds.; Elsevier: Oxford, UK, 2008; pp. 433–450. [Google Scholar]
- Lei, Z.; Huang, X. Method for Catalytic Synthesis of Lactide from Lactic Acid. CN Patent 1,721,418A, 2006. [Google Scholar]
- Sanglard, P.; Adamo, V.; Bourgeois, J.; Chappuis, T.; Vanoli, E. Poly(lactic acid) synthesis and characterization. Chim. Int. J. Chem. 2012, 66, 951–954. [Google Scholar] [CrossRef]
- Hong, C.H.; Kim, S.H.; Seo, J.Y.; Han, D.S. Manufacturing Method of Lactide from Lactic Acid. CN Patent 102,796,071A, 2012. [Google Scholar]
- Liang, S.; Wang, H.; Chen, X.; Li, F. Synthesis and purif ication of intermediate lactide. New Chem. Mater. 2005, 33, 66–70. [Google Scholar]
- Chen, J.; Cheng, C.; Wang, Y. The synthesis of lactide catalyzed by ZnO-Sn(Oct)2. Polym. Mater. Sci. Eng. 2007, 23, 74–76. [Google Scholar]
- Yoo, D.K.; Kim, D.; Lee, D.S. Synthesis of lactide from oligomeric pla: Effects of temperature, pressure, and catalyst. Macromol. Res. 2006, 14, 510–516. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L. Improved preparation of d,l-lactide from d,l-lactic acid using microwave irradiation. Polym. Bull. 2008, 61, 177–188. [Google Scholar] [CrossRef]
- Upare, P.P.; Hwang, Y.K.; Chang, J.S.; Hwang, D.W. Synthesis of lactide from alkyl lactate via a prepolymer route. Ind. Eng. Chem. Res. 2012, 51, 4837–4842. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.G.; Degée, P.; Dubois, P.; Jérôme, R. New developments on the ring opening polymerisation of polylactide. Ind. Crops Prod. 2000, 11, 265–275. [Google Scholar] [CrossRef]
- Korhonen, H.; Helminen, A.; Seppälä, J.V. Synthesis of polylactides in the presence of co-initiators with different numbers of hydroxyl groups. Polymer 2001, 42, 7541–7549. [Google Scholar] [CrossRef]
- Zhong, Z.; Dijkstra, P.J.; Feijen, J. [(salen)Al]-mediated, controlled and stereoselective ring-opening polymerization of lactide in solution and without solvent: Synthesis of highly isotactic polylactide stereocopolymers from racemic d,l-lactide. Angew. Chem. 2002, 114, 4692–4695. [Google Scholar] [CrossRef]
- Kaihara, S.; Matsumura, S.; Mikos, A.G.; Fisher, J.P. Synthesis of poly(l-lactide) and polyglycolide by ring-opening polymerization. Nat. Protoc. 2007, 2, 2767–2771. [Google Scholar] [CrossRef] [PubMed]
- Lemmouchi, Y.; Perry, M.C.; Amass, A.J.; Chakraborty, K.; Schacht, E. A novel and versatile potassium—Based catalyst for the ring opening polymerization of cyclic esters. J. Polym. Sci. A Polym. Chem. 2008, 46, 5348–5362. [Google Scholar] [CrossRef]
- Katiyar, V.; Nanavati, H. Ring-opening polymerization of L-lactide using N-heterocyclic molecules: Mechanistic, kinetics and dft studies. Polym. Chem. 2010, 1, 1491–1500. [Google Scholar] [CrossRef]
- Lohmeijer, B.G.; Pratt, R.C.; Leibfarth, F.; Logan, J.W.; Long, D.A.; Dove, A.P.; Nederberg, F.; Choi, J.; Wade, C.; Waymouth, R.M. Guanidine and amidine organocatalysts for ring-opening polymerization of cyclic esters. Macromolecules 2006, 39, 8574–8583. [Google Scholar] [CrossRef]
- Dove, A.P.; Pratt, R.C.; Lohmeijer, B.G.; Waymouth, R.M.; Hedrick, J.L. Thiourea-based bifunctional organocatalysis: Supramolecular recognition for living polymerization. J. Am. Chem. Soc. 2005, 127, 13798–13799. [Google Scholar] [CrossRef] [PubMed]
- Csihony, S.; Culkin, D.A.; Sentman, A.C.; Dove, A.P.; Waymouth, R.M.; Hedrick, J.L. Single-component catalyst/initiators for the organocatalytic ring-opening polymerization of lactide. J. Am. Chem. Soc. 2005, 127, 9079–9084. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.S.; Jardini, A.; Maciel Filho, R. Poly(lactic acid) production for tissue engineering applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Kamber, N.E.; Jeong, W.; Waymouth, R.M.; Pratt, R.C.; Lohmeijer, B.G.; Hedrick, J.L. Organocatalytic ring-opening polymerization. Chem. Rev. 2007, 107, 5813–5840. [Google Scholar] [CrossRef] [PubMed]
- Alba, A.; Schopp, A.; De Sousa Delgado, A.P.; Cherif-Cheikh, R.; Martín-Vaca, B.; Bourissou, D. Controlled ring-opening polymerization of lactide by bis-sulfonamide/amine associations: Cooperative hydrogen—Bonding catalysis. J. Polym. Sci. A Polym. Chem. 2010, 48, 959–965. [Google Scholar] [CrossRef]
- Alba, A.; du Boullay, O.T.; Martin-Vaca, B.; Bourissou, D. Direct ring-opening of lactide with amines: Application to the organo-catalyzed preparation of amide end-capped pla and to the removal of residual lactide from pla samples. Polym. Chem. 2015, 6, 989–997. [Google Scholar] [CrossRef]
- Singh, R.; Pandey, J.; Rutot, D.; Degée, P.; Dubois, P. Biodegradation of poly (ε-caprolactone)/starch blends and composites in composting and culture environments: The effect of compatibilization on the inherent biodegradability of the host polymer. Carbohydr. Res. 2003, 338, 1759–1769. [Google Scholar] [CrossRef]
- Rasal, R.M.; Hirt, D.E. Toughness decrease of pla-phbhhx blend films upon surface-confined photopolymerization. J. Biomed. Mater. Res. A 2009, 88, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. Chin. 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C. Biodegradable polymers—A review on recent trends and emerging perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Camino, G. Nanocomposites of PLA and PCL based on montmorillonite and sepiolite. Mater. Sci. Eng. C 2009, 29, 1433–1441. [Google Scholar] [CrossRef]
- Du, J.; Fang, Y.; Zheng, Y. Synthesis and characterization of poly(l-lactic acid) reinforced by biomesogenic units. Polym. Degrad. Stab. 2008, 93, 838–845. [Google Scholar] [CrossRef]
- Gruber, P.; Drumright, R.; Henton, D. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar]
- Bigg, D. Polylactide copolymers: Effect of copolymer ratio and end capping on their properties. Adv. Polym. Technol. 2005, 24, 69–82. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, C.C.; Huang, Y.Y.; Chen, K.S. Surface modification and characterization of chitosan or PLGA membrane with laminin by chemical and oxygen plasma treatment for neural regeneration. J. Biomed. Mater. Res. A 2007, 82, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, J.; Seppälä, J.V. Synthesis and characterization of lactic acid based poly(ester-amide). Macromolecules 2000, 33, 3530–3535. [Google Scholar] [CrossRef]
- Zhu, H.; Ji, J.; Shen, J. Surface engineering of poly(dl-lactic acid) by entrapment of biomacromolecules. Macromol. Rapid Commun. 2002, 23, 819–823. [Google Scholar] [CrossRef]
- Park, K.; Ju, Y.M.; Son, J.S.; Ahn, K.D.; Han, D.K. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. J. Biomater. Sci. Polym. Ed. 2007, 18, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.; Sanchez-Nacher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Leonard, D.J.; Pick, L.T.; Farrar, D.F.; Dickson, G.R.; Orr, J.F.; Buchanan, F.J. The modification of PLA and PLGA using electron-beam radiation. J. Biomed. Mater. Res. A 2009, 89, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Norazlina, H.; Kamal, Y. Graphene modifications in polylactic acid nanocomposites: A review. Polym. Bull. 2015, 72, 931–961. [Google Scholar] [CrossRef]
- Guo, C.; Xiang, M.; Dong, Y. Surface modification of poly(lactic acid) with an improved alkali-acid hydrolysis method. Mater. Lett. 2015, 140, 144–147. [Google Scholar] [CrossRef]
- Maharana, T.; Mohanty, B.; Negi, Y. Melt–solid polycondensation of lactic acid and its biodegradability. Prog. Polym. Sci. 2009, 34, 99–124. [Google Scholar] [CrossRef]
- Furlan, L.G.; Casagrande, O.L., Jr. Dual catalyst system composed by nickel and vanadium complexes containing nitrogen ligands for ethylene polymerization. J. Braz. Chem. Soc. 2005, 16, 1248–1254. [Google Scholar] [CrossRef]
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Solid-state fermentation for l-lactic acid production from agro wastes using lactobacillus delbrueckii. Process Biochem. 2006, 41, 759–763. [Google Scholar] [CrossRef]
- Pleissner, D.; Lam, W.C.; Sun, Z.; Lin, C.S.K. Food waste as nutrient source in heterotrophic microalgae cultivation. Bioresour. Technol. 2013, 137, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Kwan, T.H.; Pleissner, D.; Lau, K.Y.; Venus, J.; Pommeret, A.; Lin, C.S.K. Techno-economic analysis of a food waste valorization process via microalgae cultivation and co-production of plasticizer, lactic acid and animal feed from algal biomass and food waste. Bioresour. Technol. 2015, 198, 292–299. [Google Scholar] [CrossRef] [PubMed]




| Method | Catalyst | Solvent | T (°C) | P (kPa) | Duration (h) | Yield (%) | Mw (g·mol−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| DP | Nil | Nil | 200 | 1.37 | >100 | 34.52 | 90,000 | [9] |
| DP | Stannous chloride (0.6 mol %) | TsOH a | 200 | 4 | 0.5 | 54 | 16,000 | [10] |
| AP | Stannous octoate (0.2 wt %) | m-Xylene | 138 | Normal | 72 | - | 33,000 | [11] |
| SSP |
| TSA b | 180 | - | 5 | 99 | 36,000 | [8] |
| Nil | 130–160 | 0.07 | 30 | 68 | 202,000 | ||
| SSP |
| TSA b | 150–180 | 1.3 | 10–12 | 84 | 46,000 | [12] |
| Nil | 120–200 | 0.6 | 10–30 | 63 | 102,000 |
| Catalyst | Set up/Equipment | T (°C) | P (kPa) | Duration (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Acidified chloride catalyst supported on silica gel | Silica gel catalyst system with nitrogen gas flow | 170–260 | 0.08–10 | 4–12 | 60 | [18] |
| Stannous octoate (3 wt %) | Short path distillation | 170–250 | 0.5 | - | 95–97 | [19] |
| Zinc oxide (1 wt %) | Heating gas stream | 230–240 | 1.33–26.6 | - | 72 | [20] |
| Stannous octoate-toluene (0.04 wt %) | Oil bath with nitrogen gas flow | 220–240 | 11.3 | 6–8 | 80 | [21] |
| Zinc oxide-stannous octoate | Constant temperature heating device | 180–206 | 95 | 7 | 86.4 | [22] |
| Stannous oxide (0.1 wt %) | Oil bath | 220 | 2.67 | >8 | 77 | [23] |
| Zinc powder (1.2 wt %) | Modified domestic microwave oven | 170–180 | 3.99 | 3–5 | 40.3 | [24] |
| Stannous octoate (1 wt %) | Oil bath and ice bath | 180–210 | 1.33 | 16 | 41.3 | [25] |
| Catalyst | Solvent | T (°C) | P (kPa) | Duration (h) | Yield (%) | Mw (g·mol−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Stannous octoate (0.04 wt %) | Nil | 170 | Normal a | 2 | 91–93 | 172,663 | [19] |
| Stannous octoate | PEG b | 180–185 | Nitrogen flow | 7 min | 97–99 | 93,300 | [26] |
| Stannous octoate (0.05 mol %) | PGL-50 c | 160–200 | Nitrogen flow | 3–5 | 95–96 | 468,000 | [27] |
| Aluminum isopropoxide | Nil | 130 | Nitrogen flow | 48 | 94.8 | 24,900 | [28] |
| Stannous octoate (0.03 wt %) | 1-dodecanol | 140 | 10−3 | 10 | >95 | 100,000 | [29] |
| Potassium hexamethyl-disilazide | Toluene | 25 | - | 20 min | 100 | 98,400 | [30] |
| - | PDP d | 180–210 | 1.33 | 16 | 41.3 | 28,000 | [31] |
| TBD e (0.1 mol %) | CH2Cl2, 4-pyrenebutanol | 25 | - | 1 min | 95 | 62,600 | [32] |
| Thiourea amine (5 mol %) | CH2Cl2 | 25 | - | 105 | 98 | 42,000 | [33] |
| SIMes f | THF | 25 | - | 10 min | 87 | 16,500 | [34] |
| Novozyme 435 (10 wt %) | Ionic liquids | 90 | 4 × 10−5 | 168 | 63.2 | 37,800 | [35] |
| Method | Advantages | Disadvantages |
|---|---|---|
| Azeotropic polycondensation | Low cost | Low yield |
| Basic equipment | Low purity (usually with residual solvent and byproducts in polymer) | |
| Moderate temperature (<180 °C) | Solvent waste and pollution | |
| Solid state polycondensation | High purity (suppression of side reactions) | Low yield |
| High molecular weight | Long duration | |
| Moderate conditions | Complicated operation | |
| Ring opening polymerization | High purity | Low overall yield |
| Wide range of molecular weight (2 × 104 to 6.8 × 105 g·mol−1) [56] | Long duration | |
| Availability in high molecular weight | Demanding condition | |
| Controlled polymer properties | Complicated operation |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Daoud, W.A.; Cheuk, K.K.L.; Lin, C.S.K. Newly Developed Techniques on Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus on Poly(Lactic Acid). Materials 2016, 9, 133. https://doi.org/10.3390/ma9030133
Hu Y, Daoud WA, Cheuk KKL, Lin CSK. Newly Developed Techniques on Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus on Poly(Lactic Acid). Materials. 2016; 9(3):133. https://doi.org/10.3390/ma9030133
Chicago/Turabian StyleHu, Yunzi, Walid A. Daoud, Kevin Ka Leung Cheuk, and Carol Sze Ki Lin. 2016. "Newly Developed Techniques on Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus on Poly(Lactic Acid)" Materials 9, no. 3: 133. https://doi.org/10.3390/ma9030133
APA StyleHu, Y., Daoud, W. A., Cheuk, K. K. L., & Lin, C. S. K. (2016). Newly Developed Techniques on Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus on Poly(Lactic Acid). Materials, 9(3), 133. https://doi.org/10.3390/ma9030133