SiO2-CaO-P2O5 Bioactive Glasses: A Promising Curcuminoids Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Bioactive Glasses and Curcumininoids
2.2. Loading of Bioactive Glasses
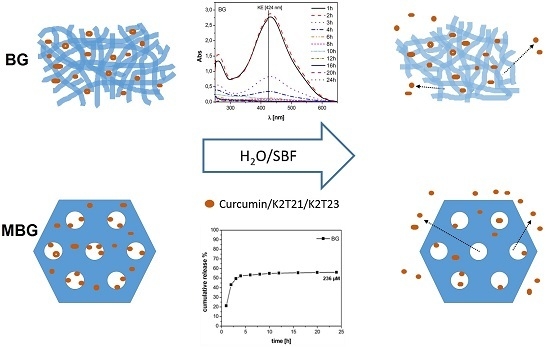
2.3. Study of the Drug Release from Bioactive Glasses
2.4. Instrumental Analysis
3. Results
3.1. Characterization of the DDSs
3.2. Static Release Study
- (i)
- the DK form is the most stable one for all the investigated curcuminoids (~300–350 nm); and
- (ii)
- degradation products show strong absorptions below 300 nm and in the range 300–350 nm.
3.3. Dynamic Release Study
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Van Gaalen, S.; Kruyt, M.; Meijer, G.; Mistry, A.; Mikos, A.; van den Beucken, J.; Jansen, J.; de Groot, K.; Cancedda, R.; Olivo, C.; et al. Chapter 19—Tissue engineering of bone. In Tissue Engineering; van Blitterswijk, C., Thomsen, P., Lindahl, A., Hubbell, J., Williams, D.F., Cancedda, R., de Bruijn, J.D., Sohier, J., Eds.; Academic Press: Burlington, MA, USA, 2008; pp. 559–610. [Google Scholar]
- Li, Y.; Liu, T.; Zheng, J.; Xu, X. Glutaraldehyde-crosslinked chitosan/hydroxyapatite bone repair scaffold and its application as drug carrier for icariin. J. Appl. Polym. Sci. 2013, 130, 1539–1547. [Google Scholar] [CrossRef]
- Mouriño, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 2010, 7, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ahn, S.-H.; Kim, G.H. Three-dimensional collagen/alginate hybrid scaffolds functionalized with a Drug Delivery System (DDS) for bone tissue regeneration. Chem. Mater. 2012, 24, 881–891. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Knowles, J.C.; Kim, H.-E. Hydroxyapatite porous scaffold engineered with biological polymer hybrid coating for antibiotic Vancomycin release. J. Mater. Sci. Mater. Med. 2005, 16, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Knowles, J.C.; Kim, H.-E. Porous scaffolds of gelatin-hydroxyapatite nanocomposites obtained by biomimetic approach: Characterization and antibiotic drug release. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Balcerzak, J.; Mucha, M. Analysis of model drug release kinetics from complex matrices of polylactide-chitosan. Prog. Chem. Appl. Chitin 2010, 15, 117–126. [Google Scholar]
- Tigani, D.; Zolezzi, C.; Trentani, F.; Ragaini, A.; Iafisco, M.; Manara, S.; Palazzo, B.; Roveri, N. Controlled release of vancomycin from cross-linked gelatine. J. Mater. Sci. Mater. Med. 2007, 19, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Hum, J.; Boccaccini, A.R. Bioactive glasses as carriers for bioactive molecules and therapeutic drugs: A review. J. Mater. Sci. Mater. Med. 2012, 23, 2317–2333. [Google Scholar] [CrossRef] [PubMed]
- Domingues, Z.R.; Cortés, M.E.; Gomes, T.A.; Diniz, H.F.; Freitas, C.S.; Gomes, J.B.; Faria, A.M.C.; Sinisterra, R.D. Bioactive glass as a drug delivery system of tetracycline and tetracycline associated with β-cyclodextrin. Biomaterials 2004, 25, 327–333. [Google Scholar] [CrossRef]
- Zhao, L.; Yan, X.; Zhou, X.; Zhou, L.; Wang, H.; Tang, J.; Yu, C. Mesoporous bioactive glasses for controlled drug release. Microporous Mesoporous Mater. 2008, 109, 210–215. [Google Scholar] [CrossRef]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R.S.R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Mouriño, V.; Cattalini, J.P.; Roether, J.A.; Dubey, P.; Roy, I.; Boccaccini, A.R. Composite polymer-bioceramic scaffolds with drug delivery capability for bone tissue engineering. Expert Opin. Drug Deliv. 2013, 10, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Knowles, J.C.; Kim, H.-E. Hydroxyapatite/poly(ε-caprolactone) composite coatings on hydroxyapatite porous bone scaffold for drug delivery. Biomaterials 2004, 25, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Yaylaoğlu, M.B.; Korkusuz, P.; Örs, Ü.; Korkusuz, F.; Hasirci, V. Development of a calcium phosphate-gelatin composite as a bone substitute and its use in drug release. Biomaterials 1999, 20, 711–719. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Calcium phosphate/chitosan composite scaffolds for controlled in vitro antibiotic drug release. J. Biomed. Mater. Res. 2002, 62, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Lemos, A.; Soundrapandian, C.; Sen, P.S.; Datta, S.; Ferreira, J.M.F.; Basu, D. Development of porous HAp and β-TCP scaffolds by starch consolidation with foaming method and drug-chitosan bilayered scaffold based drug delivery system. J. Mater. Sci. Mater. Med. 2010, 21, 2955–2969. [Google Scholar] [CrossRef] [PubMed]
- Francis, L.; Meng, D.; Knowles, J.C.; Roy, I.; Boccaccini, A.R. Multi-functional P(3HB) microsphere/45S5 Bioglass®-based composite scaffolds for bone tissue engineering. Acta Biomater. 2010, 6, 2773–2786. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Nooeaid, P.; Roether, J.A.; Schubert, D.W.; Boccaccini, A.R. Preparation and characterization of vancomycin releasing PHBV coated 45S5 Bioglass®-based glass–ceramic scaffolds for bone tissue engineering. J. Eur. Ceram. Soc. 2014, 34, 505–514. [Google Scholar] [CrossRef]
- Yao, Q.; Nooeaid, P.; Roether, J.A.; Dong, Y.; Zhang, Q.; Boccaccini, A.R. Bioglass®-based scaffolds incorporating polycaprolactone and chitosan coatings for controlled vancomycin delivery. Ceram. Int. 2013, 39, 7517–7522. [Google Scholar] [CrossRef]
- Olalde, B.; Garmendia, N.; Sáez-Martínez, V.; Argarate, N.; Nooeaid, P.; Morin, F.; Boccaccini, A.R. Multifunctional bioactive glass scaffolds coated with layers of poly(d,l-lactide-co-glycolide) and poly(n-isopropylacrylamide-co-acrylic acid) microgels loaded with vancomycin. Mater. Sci. Eng. C 2013, 33, 3760–3767. [Google Scholar] [CrossRef] [PubMed]
- López-Noriega, A.; Arcos, D.; Izquierdo-Barba, I.; Sakamoto, Y.; Terasaki, O.; Vallet-Regí, M. Ordered mesoporous bioactive glasses for bone tissue regeneration. Chem. Mater. 2006, 18, 3137–3144. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Well-ordered mesoporous bioactive glasses (MBG): A promising bioactive drug delivery system. J. Control. Release 2006, 110, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Vallet-Regí, M. Sol-gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhang, L.; He, Q.; Zhao, J.; Guo, L.; Shi, J. Mesoporous bioactive glass-coated poly(l-lactic acid) scaffolds: A sustained antibiotic drug release system for bone repairing. J. Mater. Chem. 2011, 21, 1064–1072. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Muñoz, B.; Rámila, A.; Pérez-Pariente, J.; Díaz, I.; Vallet-Regí, M. MCM-41 organic modification as drug delivery rate regulator. Chem. Mater. 2003, 15, 500–503. [Google Scholar] [CrossRef]
- Malavasi, G.; Ferrari, E.; Lusvardi, G.; Aina, V.; Fantini, F.; Morterra, C.; Pignedoli, F.; Saladini, M.; Menabue, L. The role of coordination chemistry in the development of innovative gallium-based bioceramics: The case of curcumin. J. Mater. Chem. 2011, 21, 5027–5037. [Google Scholar]
- Shruti, S.; Salinas, A.J.; Ferrari, E.; Malavasi, G.; Lusvardi, G.; Doadrio, A.L.; Menabue, L.; Vallet-Regi, M. Curcumin release from cerium, gallium and zinc containing mesoporous bioactive glasses. Microporous Mesoporous Mater. 2013, 180, 92–101. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- Basile, V.; Ferrari, E.; Lazzari, S.; Belluti, S.; Pignedoli, F.; Imbriano, C. Curcumin derivatives: Molecular basis of their anti-cancer activity. Biochem. Pharmacol. 2009, 78, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Pignedoli, F.; Imbriano, C.; Marverti, G.; Basile, V.; Venturi, E.; Saladini, M. Newly synthesized curcumin derivatives: Crosstalk between chemico-physical properties and biological activity. J. Med. Chem. 2011, 54, 8066–8077. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Atsumi, T.; Ishihara, M.; Kadoma, Y. Cytotoxicity, ROS-generation activity and radical-scavenging activity of curcumin and related compounds. Anticancer Res. 2004, 24, 563–570. [Google Scholar] [PubMed]
- Borsari, M.; Ferrari, E.; Grandi, R.; Saladini, M. Curcuminoids as potential new iron-chelating agents: Spectroscopic, polarographic and potentiometric study on their Fe(III) complexing ability. Inorg. Chim. Acta 2002, 328, 61–68. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-induced self-assembly: Nanostructures made easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- Shruti, S.; Salinas, A.J.; Malavasi, G.; Lusvardi, G.; Menabue, L.; Ferrara, C.; Mustarelli, P.; Vallet-Regì, M. Structural and in vitro study of cerium, gallium and zinc containing sol-gel bioactive glasses. J. Mater. Chem. 2012, 22, 13698–13706. [Google Scholar] [CrossRef]
- Salinas, A.J.; Shruti, S.; Malavasi, G.; Menabue, L.; Vallet-Regí, M. Substitutions of cerium, gallium and zinc in ordered mesoporous bioactive glasses. Acta Biomater. 2011, 7, 3452–3458. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982. [Google Scholar]
- Nardo, L.; Maspero, A.; Selva, M.; Bondani, M.; Palmisano, G.; Ferrari, E.; Saladini, M. Excited-state dynamics of bis-dehydroxycurcumin carboxylic acid, a water-soluble derivative of the photosensitizer curcumin. J. Phys. Chem. A 2012, 116, 9321–9330. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, V.; Varini, E.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Lusvardi, G.; Pedone, A.; Benedetti, F.; Luches, P. The effect of composition on structural, thermal, redox and bioactive properties of Ce-containing glasses. Mater. Des. 2016, 97, 73–85. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]






| Na+ | K+ | Ca2+ | Mg2+ | Cl− | HPO42− | HCO3− | SO42− | |
|---|---|---|---|---|---|---|---|---|
| Plasma | 142.0 | 5.0 | 2.5 | 1.5 | 103.0 | 1.0 | 27.0 | 0.5 |
| SBF | 142.0 | 5.7 | 2.5 | 1.5 | 147.8 | 1.0 | 4.2 | 0.5 |
| Glass | Drug | %drug (w/w) ± SD |
|---|---|---|
| BG | Curcumin | 3.1 ± 0.1 |
| K2T21 | 3.3 ± 0.1 | |
| K2T23 | 2.3 ± 0.2 | |
| MBG | Curcumin | 3.9 ± 0.2 |
| K2T21 | 3.1 ± 0.1 | |
| K2T23 | 2.4 ± 0.1 |
| SSA (m2/g) | BJH Mesopore Area (m2/g) | BJH Average Pore Width (Å) | |
|---|---|---|---|
| BG before | 120 | 10 | – |
| BG after | 93 | 3 | – |
| MBG before | 472 | 224 | 30 |
| MBG after | 359 | 162 | 30 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolini, V.; Caselli, M.; Ferrari, E.; Menabue, L.; Lusvardi, G.; Saladini, M.; Malavasi, G. SiO2-CaO-P2O5 Bioactive Glasses: A Promising Curcuminoids Delivery System. Materials 2016, 9, 290. https://doi.org/10.3390/ma9040290
Nicolini V, Caselli M, Ferrari E, Menabue L, Lusvardi G, Saladini M, Malavasi G. SiO2-CaO-P2O5 Bioactive Glasses: A Promising Curcuminoids Delivery System. Materials. 2016; 9(4):290. https://doi.org/10.3390/ma9040290
Chicago/Turabian StyleNicolini, Valentina, Monica Caselli, Erika Ferrari, Ledi Menabue, Gigliola Lusvardi, Monica Saladini, and Gianluca Malavasi. 2016. "SiO2-CaO-P2O5 Bioactive Glasses: A Promising Curcuminoids Delivery System" Materials 9, no. 4: 290. https://doi.org/10.3390/ma9040290
APA StyleNicolini, V., Caselli, M., Ferrari, E., Menabue, L., Lusvardi, G., Saladini, M., & Malavasi, G. (2016). SiO2-CaO-P2O5 Bioactive Glasses: A Promising Curcuminoids Delivery System. Materials, 9(4), 290. https://doi.org/10.3390/ma9040290