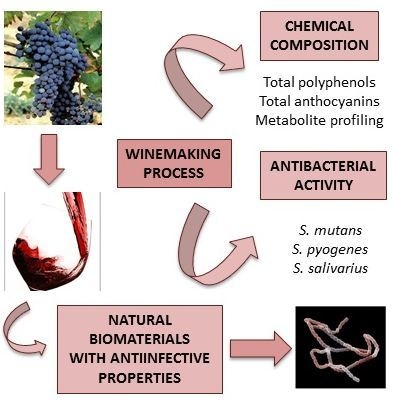
Effect of Winemaking on the Composition of Red Wine as a Source of Polyphenols for Anti-Infective Biomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Wine Samples and Winemaking Process
2.3. Total Polyphenol Content
2.4. Total Monomeric Anthocyanin Content
2.5. HPLC-PDA-ESI-MSn Analysis
2.6. Bacterial Strains
2.7. Evaluation of Minimum Inhibitory Concentration (MIC)
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CLSI | Clinical and Laboratory Standards Institute |
| CGE | Cyanidin-3-glucoside equivalents |
| GAE | Gallic acid equivalents |
| MIC | Minimum inhibitory concentration |
| NCCLS | National Committee for Clinical Laboratory Standards |
| TMA | Total monomeric anthocyanins |
| TPC | Total polyphenol content |
| YAN | Yeast assimilable nitrogen |
References
- Leung, E.; Weil, D.E.; Raviglione, M.; Nakatani, H. The WHO policy package to combat antimicrobial resistance. Bull. World Health Organ. 2011, 89, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants belonging to the genus Thymus as antibacterial agents: From farm to pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lu, Y.; Cui, H.; Jia, X.; Bai, H.; Ma, Y. Factors affecting the physical properties of edible composite film prepared from zein and wheat gluten. Molecules 2012, 17, 3794–3804. [Google Scholar] [CrossRef] [PubMed]
- Moreira Mdel, R.; Pereda, M.; Marcovich, N.E.; Roura, S.I. Antimicrobial effectiveness of bioactive packaging materials from edible chitosan and casein polymers: Assessment on carrot, cheese, and salami. J. Food Sci. 2011, 76, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Péroval, C.; Debeaufort, F.; Despré, D.; Voilley, A. Edible arabinoxylan-based films. 1. Effects of lipid type on water vapor permeability, film structure, and other physical characteristics. J. Agric. Food Chem. 2002, 50, 3977–3983. [Google Scholar] [CrossRef] [PubMed]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.; Gregoire, S.; Singh, A.P.; Vorsa, N.; Schaich, K.; Bowen, W.H.; Koo, H. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol. Lett. 2006, 257, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Percival, R.S.; Devine, D.A.; Duggal, M.S.; Chartron, S.; Marsh, P.D. The effect of cocoa polyphenols on the growth, metabolism, and biofilm formation by Streptococcus mutans and Streptococcus sanguinis. Eur. J. Oral Sci. 2006, 114, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Sureda, A.; Daglia, M.; Rezaei, P.; Nabavi, S.M. Anti-Oxidative Polyphenolic Compounds of Cocoa. Curr. Pharm. Biotech. 2015, 16, 891–901. [Google Scholar] [CrossRef]
- Marchese, A.; Coppo, E.; Sobolev, A.P.; Rossi, D.; Mannina, L.; Daglia, M. Influence of in vitro simulated gastroduodenal digestion on the antibacterial activity, metabolic profiling and polyphenols content of green tea (Camellia sinensis). Food Res. Int. 2014, 63, 182–191. [Google Scholar] [CrossRef]
- Daglia, M.; Papetti, A.; Grisoli, P.; Aceti, C.; Dacarro, C.; Gazzani, G. Antibacterial activity of red and white wine against oral streptococci. J. Agric. Food Chem. 2007, 55, 5038–5042. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Zhao, Y. Physicochemical, nutritional, and antimicrobial properties of wine grape (cv. Merlot) pomace extract-based films. J. Food Sci. 2011, 76, 309–317. [Google Scholar] [CrossRef] [PubMed]
- López-Vélez, M.; Martínez-Martínez, F.; Valle-Ribes, C.D. The Study of Phenolic Compounds as Natural Antioxidants in Wine. Crit. Rev. Food Sci. Nutr. 2003, 43, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Sulc, M.; Faitová, K.; Pivec, V. Major factors influencing antioxidant contents and antioxidant activity in grapes and wines. Int. J. Wine Res. 2009, 101, 101–121. [Google Scholar] [CrossRef]
- Kanner, J.; Frankel, E.; Granit, R.; German, B.; Kinsella, J.E. Natural antioxidants in grapes and wines. J. Agric. Food Chem. 1994, 42, 64–69. [Google Scholar] [CrossRef]
- Pereira, V.; Albuquerque, F.; Cacho, J.; Marques, J. Polyphenols, Antioxidant Potential and Color of Fortified Wines during Accelerated Ageing: The Madeira Wine Case Study. Molecules 2013, 18, 2997–3017. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Scrocco, C.; Sepielli, G.; Del Nobile, M.A. Wine Processing: A Critical Review on Physical, Chemical, and Sensory Implications of Innovative Vinification Procedures. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Baranac, J.M.; Petranoviv, N.A.; Dimitiric-Markovic, J.M. Spectrophotometric study of anthocyan copigmentation reactions. 2. Malvin and the nonglycosidized flavone quercetin. J. Agric. Food Chem. 1997, 45, 1694–1697. [Google Scholar] [CrossRef]
- Soto Vázquez, E.; Río Segade, S.; Orriols Fernández, I. Effect of the winemaking technique on phenolic composition and chromatic characteristics in young red wines. Eur. Food Res. Technol. 2010, 231, 789–802. [Google Scholar] [CrossRef]
- Escot, S.; Feuillat, M.; Dulau, L.; Charpentier, C. Release of polysaccharides by yeast and the influence of polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Cano-López, M. A review on micro-oxygenation of red wines: Claims, benefits and the underlying chemistry. Food Chem. 2011, 125, 1131–1140. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Micro-oxygenation and oak chip treatments of red wines: Effects on colour-related phenolics, volatile composition and sensory characteristics. Part I: Petit Verdot wines. Food Chem. 2011, 124, 727–737. [Google Scholar] [CrossRef]
- Carillo, M.; Formato, A.; Fabiani, A.; Scaglione, G.; Pucillo, G.P. An inertizing and cooling process for grapes cryomaceration. Electron. J. Biotechnol. 2011, 14, 7–14. [Google Scholar] [CrossRef]
- Fretté, X.C.; Hansen, J.H.; Raasthøj, J.C.; Broe, J.; Christensen, L.P. Content of selected phenolic compounds in wine from rondo grapes grown in Denmark and effect of heat and cryomaceration. Planta Med. 2012, 78, 1281. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Grieco, F. Functional Properties of Grape and Wine Polyphenols. Plant Foods Hum. Nutr. 2015, 70, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Habtemariam, S.; Daglia, M.; Shafighi, N.; Barber, A.J.; Nabavi, S.M. Anthocyanins as a potential therapy for diabetic retinopathy. Curr. Med Chem. 2015, 22, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.-P.; Nettleton, J.A.; Jacobs, D.R. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [PubMed]
- Soobrattee, M.A.; Bahorun, T.; Aruoma, O.I. Chemopreventive actions of polyphenolic compounds in cancer. Biofactors 2006, 27, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leifert, W.R.; Abeywardena, M.Y. Cardioprotective actions of grape polyphenols. Nutr. Res. 2008, 28, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Côté, C.D.; Rasmussen, B.A.; Duca, F.A.; Zadeh-Tahmasebi, M.; Baur, J.A.; Daljeet, M.; Breen, D.M.; Filippi, B.M.; Lam, T.K.T. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat. Med. 2015, 21, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Smith, K.; Labinskyy, N.; Orosz, Z.; Rivera, A.; Ungvari, Z. Resveratrol attenuates TNF-alpha-induced activation of coronary arterial endothelial cells: Role of NF-kappaB inhibition. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1694–H1699. [Google Scholar] [CrossRef] [PubMed]
- Barbosa da Silva, A.C.; Cruz, J.D.S.; Sampaio, F.C.; Machado de Araujo, D.A. Detection of oral streptococci in dental biofilm from caries-active and caries-free children. Braz. J. Microbiol. 2008, 39, 648–651. [Google Scholar]
- Lemos, J.A.; Abranches, J.; Burne, R.A. Responses of cariogenic streptococci to environmental stresses. Curr. Issues Mol. Biol. 2005, 7, 95–107. [Google Scholar] [PubMed]
- Stauder, M.; Papetti, A.; Mascherpa, D.; Schito, A.M.; Gazzani, G.; Pruzzo, C.; Daglia, M. Antiadhesion and antibiofilm activities of high molecular weight coffee components against Streptococcus mutans. J. Agric. Food Chem. 2010, 58, 11662–11666. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, I.; Thurnheer, T.; Bartolomé, B.; Moreno-Arribas, M.V. Red Wine and Oenological Extracts Display Antimicrobial Effects in an Oral Bacteria Biofilm Model. J. Agric. Food Chem. 2014, 62, 4731–4737. [Google Scholar] [CrossRef] [PubMed]
- Zoellner, H. Dental infection and vascular disease. Semin. Thromb. Hemost. 2011, 37, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Bandela, V.; Munagapati, B.; Karnati, R.K.; Venkata, G.R.; Nidudhur, S.R. Osteoporosis: Its Prosthodontic Considerations. A Review. J. Clin. Diagn. Res. 2015, 9, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Leech, M.T.; Bartold, P.M. The association between rheumatoid arthritis and periodontitis. Best Pract. Res. Clin. Rheumatol. 2015, 29, 189–201. [Google Scholar] [PubMed]
- Ehrlich, G.D.; Hu, F.Z.; Sotereanos, N.; Sewicke, J.; Parvizi, J.; Nara, P.L.; Arciola, C.R. What role do periodontal pathogens play in osteoarthritis and periprosthetic joint infections of the knee? J. Appl. Biomater. Funct. Mater. 2014, 12, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Lagerweij, M.D.; van Loveren, C. Declining Caries Trends: Are We Satisfied? Curr. Oral Health Rep. 2015, 4, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Sadat Sajadi, F.; Moradi, M.; Pardakhty, A.; Yazdizadeh, R.; Madani, F. Effect of Fluoride, Chlorhexidine and Fluoride-chlorhexidine Mouthwashes on Salivary Streptococcus mutans Count and the Prevalence of Oral Side Effects. J. Dent. Res. Dent. Clin. Dent. Prospects 2015, 9, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Van Loveren, C.; Broukal, Z.; Oganessian, E. Functional foods/ingredients and dental caries. Eur. J. Nutr. 2012, 51, S15–S25. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Neves, A.C.; Fernandes, T.A.; Fernandes, A.L.; Mateus, N.; De Freitas, V.; Leandro, C.; Spranger, M.I. Evolution of phenolic composition of red wine during vinification and storage and its contribution to wine sensory properties and antioxidant activity. J. Agric. Food Chem. 2011, 59, 6550–6557. [Google Scholar] [CrossRef] [PubMed]
- Ginjom, I.R.; D’Arcy, B.R.; Caffin, N.A.; Gidley, M.J. Phenolic Contents and Antioxidant Activities of Major Australian Red Wines throughout the Winemaking Process. J. Agric. Food Chem. 2010, 58, 10133–10142. [Google Scholar] [CrossRef] [PubMed]
- Preserova, J.; Ranc, V.; Milde, D.; Kubistova, V.; Stavek, J. Study of phenolic profile and antioxidant activity in selected Moravian wines during winemaking process by FT-IR spectroscopy. J. Food Sci. Technol. 2015, 52, 6405–6414. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arribas, M.V.; Gómez-Cordovés, C.; Martín-Álvarez, P.J. Evolution of red wine anthocyanins during malolactic fermentation, postfermentative treatments and ageing with lees. Food Chem. 2008, 109, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Arcidiaco, P.; Nabavi, S.M.; Daglia, M. Antidepressive-like effects and antioxidant activity of green tea and GABA green tea in a mouse model of post-stroke depression. Mol. Nutr. Food Res. 2015, 60, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [PubMed]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Eighth Edition; M07-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009.
- Malekinejad, H.; Bazargani-Gilani, B.; Tukmechi, A.; Ebrahimi, H. A cytotoxicity and comparative antibacterial study on the effect of Zataria multiflora Boiss, Trachyspermum copticum essential oils, and Enrofloxacin on Aeromonas hydrophila. Avicenna J. Phytomed. 2012, 2, 188–195. [Google Scholar] [PubMed]
- Ivanova, V.; Vojnoski, B.; Stefova, M. Effect of the Winemaking Practices and Aging on Phenolic Content of Smederevka and Chardonnay Wines. Food Bioprocess Technol. 2011, 4, 1512–1518. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Trattato di Enologia II, Chimica del vino, Stabilizzazione, Trattamenti; Ribereau-Gayon, P., Glories, Y., Maujean, A., Dubourdieu, D., Eds.; Agricole: Bologna, Italy, 1998. [Google Scholar]
- Borazan, A.A.; Bozan, B. The influence of pectolytic enzyme addition and prefermentative mash heating during the winemaking process on the phenolic composition of Okuzgozu red wine. Food Chem. 2013, 138, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Soares, D.C.; Nascimento, M.T.; Pinto-da-Silva, L.H.; Sarzedas, G.C.; Tinoco, L.W.; Saraiva, E.M. Resveratrol is active against Leishmania amazonensis: In vitro effect of its association with Amphotericin B. Antimicrob. Agents Chemother. 2014, 58, 6197–6208. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Muñoz, N.; Gomez-Alonso, S.; Garcia-Romero, E.; Hermosin-Gutierrez, I. Flavonol profiles of Vitis Vinifera red grapes and their single-cultivar wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Riihinen, K.R.; Ou, Z.M.; Godecke, T.; Lankin, D.C.; Pauli, G.F.; Wu, C.D. The antibiofilm activity of lingonberry flavonoids against oral pathogens is a case connected to residual complexity. Fitoterapia 2014, 97, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Zulian, C.; Nicolini, G.; Valenti, L. Wine, biodiversity, technology and antioxidants. Ann. N. Y. Acad. Sci. 2002, 957, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Habtemariam, S.; Nabavi, S.F.; Sureda, A.; Daglia, M.; Moghaddam, A.H.; Amani, M.A. Protective effect of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress in rat’s kidney. Mol. Cell. Biochem. 2013, 372, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Talas, Z.S.; Nabavi, S.M. Polyphenols: Well beyond the antioxidant capacity: Gallic acid and related compounds as neuroprotective agents: You are what you eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Choubey, S.; Varughese, L.R.; Kumar, V.; Beniwal, V. Medicinal importance of gallic acid and its ester derivatives: A patent review. Pharm. Patent Anal. 2015, 4, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Saini, R.; Dangwal, K.; Singh, H.; Garg, V. Antioxidant and antiproliferative activities of phenolics isolated from fruits of Himalayan yellow raspberry (Rubus ellipticus). J. Food Sci. Technol. 2014, 51, 3369–3375. [Google Scholar] [CrossRef] [PubMed]
- Lupoae, P.; Cristea, V.; Borda, D.; Lupoae, M.; Gurau, G.; Dinica, R.M. Phytochemical Screening: Antioxidant and Antibacterial Properties of Potamogeton Species in Order to Obtain Valuable Feed Additives. J. Oleo Sci. 2015, 64, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.; Messersmith, P.B. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew. Chem. Int. Ed. 2013, 52, 10766–10770. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]



| Wine Sample | Collection Date | Winemaking Step | Abbreviation |
|---|---|---|---|
| Croatina | 30 September 2015 | Grape crushing, addition of yeast and K2S2O5 | CR1 |
| Croatina | 2 October 2015 | Alcoholic fermentation | CR2 |
| Croatina | 6 October 2015 | Alcoholic fermentation | CR3 |
| Croatina | 8 October 2015 | Malolactic fermentation | CR4 |
| Croatina | 12 October 2015 | Malolactic fermentation | CR5 |
| Croatina | 19 October 2015 | Malolactic fermentation | CR6 |
| Croatina | 26 October 2015 | Malolactic fermentation | CR7 |
| Croatina | 27 October 2015 | Malolactic fermentation | CR8 |
| Barbera | 27 October 2015 | Wine used to dilute Croatina | BA |
| Croatina + Barbera before SO2 addition | 27 October 2015 | Addition of Barbera to obtain Bonarda wine | CB1 |
| Croatina + Barbera after SO2 addition | 27 October 2015 | SO2 addition | CB2 |
| Bonarda wine (end of fermentation) | 30 October 2015 | End of fermentation | BO |
| Croatina Torchiato | 8 October 2015 | Marc pressing | CT1 |
| Croatina Torchiato | 12 October 2015 | Fermentation | CT2 |
| Croatina Torchiato | 19 October 2015 | Fermentation | CT3 |
| Peak Number | Retention Time (RT) (min) | λ Max (nm) | m/z | HPLC-ESI-MSn (% of Base Peak) | Proposed Structure |
|---|---|---|---|---|---|
| Organic and benzoic acids | |||||
| 1 | 12.0 | 203, 214 | 191 | 111 (100) | citric acid |
| 2 | 28.0 | 221, 269 | 169 | 125 (100) | gallic acid (a) |
| Flavonols | |||||
| 3 | 35.4 | 214, 245, 286 | 493 | 331 (100) | laricitrin-hexoside (a) |
| 15 | 74.9 | 214, 233, 278 | 447 | 401 (100), 285 (100) | kaempferol-hexoside |
| 27 | 118.3 | 226, 268, 328 | 479 | 316 (100), 317 (30) | myricetin-hexoside |
| 32 | 121.8 | 226, 268, 285 | 463 | 301 (100) | quercetin-hexoside (b) |
| 35 | 123.8 | 226, 312, 348 | 507 | 344 (100), 345 (50) | syringetin-hexoside (b) |
| 39 | 126.1 | 226, 255, 349 | 477 | 301 (100) | quercetin-glucuronide |
| Flavan-3-ols | |||||
| 4 | 36.4 | 207, 269 | 609 | 441 (100), 423 (85), 305 (35) | gallocatechin derivative |
| 7 | 48.0 | 207, 275 | 305 | 179 (100), 221 (80), 219 (80), 261 (70) | gallocatechin (c), (d) |
| 12 | 69.0 | 224, 230, 279 | 289 | 245 (100), 205 (40), 179 (20) | catechin |
| 20 | 85.8 | 228, 279 | 289 | 246 (100), 205 (40), 179 (10) | epicatechin (c), (d) |
| Tannins | |||||
| 5 | 39.7 | 219, 278 | 865 | 695 (100), 577 (40), 289 (20), 407 (10) | procyanidin trimer |
| 6 | 44.9 | 218, 270 | 331 | 169 (100) | galloylglucose (a), (c), (e) |
| 9 | 62.6 | 279 | 577 | 425 (100), 407 (40), 289 (20) | procyanidin dimer |
| 11 | 67.5 | 224, 278 | 577 | 425 (100), 407 (60), 289 (20) | procyanidin dimer |
| 13 | 69.6 | 224, 279 | 865 | 695 (100), 577 (80), 407 (25), 298 (20) | procyanidin trimer |
| 16 | 75.5 | 278 | 865 | 695 (100), 577 (80), 407 (25), 298 (20) | procyanidin trimer |
| 18 | 79.6 | 221, 278 | 577 | 425 (100), 407 (50), 289 (15) | procyanidin dimer |
| 22 | 89.8 | 223, 280 | 865 | 695 (100), 577 (40), 793 (40) | procyanidin trimer (f) |
| Anthocyanins (g) | |||||
| 8 | 54.5 | 223, 276 | 595 | 443 (100), 425 (80) | cyanidin-6-O-coumaroylhexoside |
| 10 | 64.0 | 215, 222, 279 | 465 | 303 (100) | delphinidin-hexoside |
| 14 | 74.0 | 206, 279 | 449 | 287 (100) | cyanidin-hexoside |
| 17 | 79.4 | 205, 214, 279 | 479 | 317 (100) | petunidin-hexoside |
| 19 | 81.1 | 226, 280 | 463 | 301 (100) | peonidin-hexoside |
| 21 | 88.0 | 223, 279 | 493 | 331 (100) | malvidin-hexoside |
| 23 | 93.0 | 224, 280 | 507 | 303 (100) | delphinidin-3-acetylhexoside (h), (i) |
| 24 | 104.0 | 206, 219, 280 | 521 | 317 (100) | petunidin-3-acetylhexoside |
| 25 | 107.5 | 204, 215, 281 | 517 | 355 (100) | malvidin-3-glucosylacetaldehyde |
| 26 | 111.0 | 224, 280 | 535 | 331 (100) | malvinidin-3-acetylhexoside |
| 29 | 120.0 | 233, 280 | 809 | 357 (100), 519 (80), 547 (20) | malvidin-3-O-glucosyl-8-ethyl-epicatechin |
| 30 | 121.1 | 232, 280 | 561 | 399 (100) | malvidin-3-glucosidepiruvate |
| 31 | 121.3 | 230, 283 | 625 | 317 (100) | petunidin-3-(6-O-coumaroyl)-hexoside (l) |
| 33 | 122.0 | 230, 280 | 609 | 447 (100), 301 (70) | peonidin-3-O-(C6-coumaroyl)-hexoside (l) |
| 34 | 122.2 | 232, 282 | 639 | 331 (100) | malvidin-3-coumaroylhexoside |
| 36 | 124.0 | 227, 280 | 707 | 399 (100) | malvidin-3-O-coumaroylglucoside pyruvate |
| 37 | 124.5 | 227, 280 | 707 | 399 (100) | carboxypyrano-malvidin-3-coumaroylglucoside (d) |
| 38 | 125.9 | 226, 355 | 479 | 303 (100) | delphinidin-glucuronide |
| Stilbenoids | |||||
| 28 | 119.6 | - | 389 | 227 (100) | resveratrol-hexoside (b) |
| Flavonols | CR1 | CR2 | CR3-CR8 | BA | CB1-CB2 | BO | CT1 | CT2 | CT3 |
| laricitrin-hexoside | - | + | + | + | + | + | + | + | + |
| kaempferol-hexoside | + | + | + | + | + | + | + | + | + |
| myricetin-hexoside | + | + | + | + | + | + | + | + | + |
| quercetin-hexoside | + | + | + | - | + | + | + | + | + |
| quercetin-glucuronide | + | + | + | + | + | + | + | + | + |
| syringetin-hexoside | + | + | + | - | + | + | + | + | + |
| Flavan-3-ols | CR1 | CR2 | CR3-CR8 | BA | CB1-CB2 | BO | CT1 | CT2 | CT3 |
| gallocatechin derivative | + | + | + | + | + | + | + | + | + |
| gallocatechin | - | - | + | + | + | + | - | - | - |
| catechin | + | + | + | + | + | + | + | + | + |
| epicatechin | - | - | + | + | + | + | - | - | - |
| Tannins | CR1 | CR2 | CR3-CR8 | BA | CB1-CB2 | BO | CT1 | CT2 | CT3 |
| galloylglucose | - | - | + | - | + | + | + | + | - |
| procyanidin dimer | + | + | + | + | + | + | + | + | + |
| procyanidin dimer | + | + | + | + | + | + | + | + | + |
| procyanidin dimer | + | + | + | + | + | + | + | + | + |
| procyanidin trimer | + | + | + | + | + | + | + | + | + |
| procyanidin trimer | + | + | + | + | + | + | + | + | + |
| procyanidin trimer | + | + | + | + | + | + | + | + | + |
| procyanidin trimer | - | - | - (CR3-CR6) | + | + | + | + | + | + |
| Stilbenoids | CR1 | CR2 | CR3-CR8 | BA | CB1-CB2 | BO | CT1 | CT2 | CT3 |
| resveratrol-hexoside | + | + | + | - | + | + | + | + | + |
| Organic and Benzoic Acids | CR1 | CR2 | CR3-CR8 | BA | CB1-CB2 | BO | CT1 | CT2 | CT3 |
| citric acid | + | + | + | + | + | + | + | + | + |
| gallic acid | - | + | + | + | + | + | + | + | + |
| Anthocyanin | CR1 | CR2 | CR3-CR8 | BA | CB1-CB2 | BO | CT1 | CT2 | CT3 |
|---|---|---|---|---|---|---|---|---|---|
| cyanidin-6-O-coumaroylhexoside | + | + | + | + | + | + | + | + | + |
| cyanidin-hexoside | + | + | + | + | + | + | + | + | + |
| delphinidin-hexoside | + | + | + | + | + | + | + | + | + |
| delphinidin-3-acetylhexoside | in trace | in trace | in trace from CR3 to CR6 | + | + | + | - | - | + |
| delphinidin-glucuronide | + | + | + | + | + | + | + | + | + |
| petunidin-hexoside | + | + | + | + | + | + | + | + | + |
| petunidin-3-acetylhexoside | + | + | + | + | + | + | + | + | + |
| petunidin-3-(6-O-coumaroyl)-hexoside | in trace | ||||||||
| peonidin-hexoside | + | + | + | + | + | + | + | + | + |
| peonidin-3-(C6-coumaroyl)-hexoside | in trace | ||||||||
| malvidin-hexoside | + | + | + | + | + | + | + | + | + |
| malvidin-3-glucosylacetaldehyde | + | + | + | + | + | + | + | + | + |
| malvidin-3-acetylhexoside | + | + | + | + | + | + | + | + | + |
| malvidin-3-O-glucosyl-8-ethyl-epicatechin | + | + | + | + | + | + | + | + | + |
| malvidin-3-glucosidepiruvate | + | + | + | + | + | + | + | + | + |
| malvidin-3-O-coumaroylglucoside pyruvate | + | + | + | + | + | + | + | + | + |
| malvidin-3-coumaroylhexoside | + | + | + | + | + | + | + | + | + |
| carboxypyrano-malvidin-3-courmaroylglucoside | + | + | + | + | + | + | - | - | - |
| Delacoholized Wine | S. mutans 35176 MIC (v/v %) | S. salivarius 11878 MIC (v/v %) | S. pyogenes BIO1926 MIC (v/v %) |
|---|---|---|---|
| CR1 | 60 | 60 | 60 |
| CR2 | 40 | 40 | 40 |
| CR3 | 20 | 20 | 40 |
| CR4 | 20 | 20 | 40 |
| CR5 | 20 | 20 | 40 |
| CR6 | 20 | 20 | 40 |
| CR7 | 20 | 20 | 40 |
| CR8 | 20 | 20 | 40 |
| BA | 40 | 40 | 40 |
| CB1 | 20 | 20 | 40 |
| CB2 | 20 | 20 | 40 |
| BO | 20 | 20 | 20 |
| CT1 | 20 | 20 | 20 |
| CT2 | 20 | 20 | 20 |
| CT3 | 20 | 20 | 20 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lorenzo, A.; Bloise, N.; Meneghini, S.; Sureda, A.; Tenore, G.C.; Visai, L.; Arciola, C.R.; Daglia, M. Effect of Winemaking on the Composition of Red Wine as a Source of Polyphenols for Anti-Infective Biomaterials. Materials 2016, 9, 316. https://doi.org/10.3390/ma9050316
Di Lorenzo A, Bloise N, Meneghini S, Sureda A, Tenore GC, Visai L, Arciola CR, Daglia M. Effect of Winemaking on the Composition of Red Wine as a Source of Polyphenols for Anti-Infective Biomaterials. Materials. 2016; 9(5):316. https://doi.org/10.3390/ma9050316
Chicago/Turabian StyleDi Lorenzo, Arianna, Nora Bloise, Silvia Meneghini, Antoni Sureda, Gian Carlo Tenore, Livia Visai, Carla Renata Arciola, and Maria Daglia. 2016. "Effect of Winemaking on the Composition of Red Wine as a Source of Polyphenols for Anti-Infective Biomaterials" Materials 9, no. 5: 316. https://doi.org/10.3390/ma9050316
APA StyleDi Lorenzo, A., Bloise, N., Meneghini, S., Sureda, A., Tenore, G. C., Visai, L., Arciola, C. R., & Daglia, M. (2016). Effect of Winemaking on the Composition of Red Wine as a Source of Polyphenols for Anti-Infective Biomaterials. Materials, 9(5), 316. https://doi.org/10.3390/ma9050316