Effective Degradation of Aqueous Tetracycline Using a Nano-TiO2/Carbon Electrocatalytic Membrane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Composition and Structure of Nano-TiO2/Carbon Membrane
2.2. Performance of Nano-TiO2/Carbon Membrane in Degradation of TC Solution
2.2.1. Effect of Current Density
2.2.2. Effect of Temperature
2.2.3. Effect of Residence Time
2.2.4. Effect of TC Initial Concentration
2.2.5. Effect of pH
2.2.6. Effect of Operation Time
3. Experimental Section
3.1. Materials and Reagents
3.2. Preparation and Characterization of the Nano-TiO2/Carbon Membrane
3.3. Evaluation of TC Removal Performance
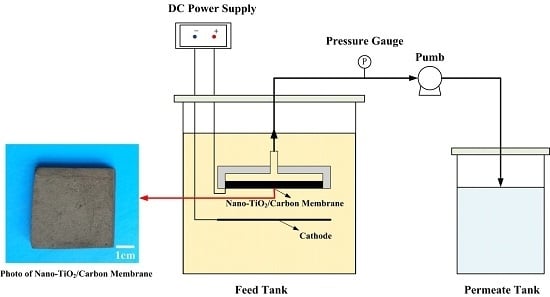
3.3.1. Electrocatalytic Membrane Assembly Filter
3.3.2. TC Removal Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AOPs | Advanced Oxidation Processes |
| ECMR | Electrocatalytic Membrane Reactor |
| COD | Chemical Oxygen Demand |
| SEM | Electron Microscopy |
| EDS | Energy Dispersive Spectroscopy |
| XRD | X-ray Diffraction |
| XPS | X-ray Photoelectron Spectroscopy |
| HPLC | High Performance Liquid Chromatography |
| RT | Residence Time |
| TC | Tetracycline |
| NF | Nanofiltration |
| RO | Reverse Osmosis |
References
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water: A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Oulton, R.L.; Kohn, T.; Cwiertny, D.M. Pharmaceuticals and personal care products in effluent matrices: A survey of transformation and removal during wastewater treatment and implications for wastewater management. J. Environ. Monitor. 2010, 12, 1956–1978. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A Review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Khetan, S.K.; Collins, T.J. Human pharmaceuticals in the aquatic environment: A challenge to green chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.; Murby, E.; Costanzo, S. Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recycling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Mompelat, S.; le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- De Godos, I.; Muñoz, R.; Guieysse, B. Tetracycline removal during wastewater treatment in high-rate algal ponds. J. Hazard. Mater. 2012, 229, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Eichhorn, P.; Jensen, J.N.; Weber, A.S.; Aga, D.S. Removal of antibiotics in wastewater: Effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environ. Sci. Technol. 2005, 39, 5816–5823. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Q.; Liu, Y.; Guo, Z.; Shao, L. Nanofiltration membrane achieving dual resistance to fouling and chlorine for “green” separation of antibiotics. J. Membr. Sci 2015, 493, 156–166. [Google Scholar] [CrossRef]
- Guo, R.; Chen, J. Application of alga-activated sludge combined system (AASCS) as a novel treatment to remove cephalosporins. Chem. Eng. J. 2015, 260, 550–556. [Google Scholar] [CrossRef]
- Ren, A.; Liu, C.; Hong, Y.; Shi, W.; Lin, S.; Li, P. Enhanced visible-light-driven photocatalytic activity for antibiotic degradation using magnetic NiFe2O4/Bi2O3 heterostructures. Chem. Eng. J. 2014, 258, 301–308. [Google Scholar] [CrossRef]
- Sun, P.; Pavlostathis, S.G.; Huang, C.-H. Photodegradation of veterinary ionophore antibiotics under UV and solar irradiation. Environ. Sci. Technol. 2014, 48, 13188–13196. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Song, W.; Cooper, W.J.; Jung, J.; Greaves, J. Degradation of tetracycline antibiotics: Mechanisms and kinetic studies for advanced oxidation/reduction processes. Chemosphere 2010, 78, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Miralles-Cuevas, S.; Arqués, A.; Maldonado, M.; Sánchez-Pérez, J.; Rodríguez, S.M. Combined nanofiltration and photo-fenton treatment of water containing micropollutants. Chem. Eng. J. 2013, 224, 89–95. [Google Scholar] [CrossRef]
- Beltran, F.J.; Aguinaco, A.; García-Araya, J.F.; Oropesa, A. Ozone and photocatalytic processes to remove the antibiotic sulfamethoxazole from water. Water Res. 2008, 42, 3799–3808. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, H.; Feng, Y.; Yang, F.; Zhang, J. Removal of trace antibiotics from wastewater: A systematic study of nanofiltration combined with ozone-based advanced oxidation processes. Chem. Eng. J. 2014, 240, 211–220. [Google Scholar] [CrossRef]
- Shah, A.D.; Huang, C.-H.; Kim, J.-H. Mechanisms of antibiotic removal by nanofiltration membranes: Model development and application. J. Membr. Sci. 2012, 389, 234–244. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Duan, L.; Zhu, D. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Miyata, M.; Ihara, I.; Yoshid, G.; Toyod, K.; Umetsu, K. Electrochemical oxidation of tetracycline antibiotics using a Ti/IrO2 anode for wastewater treatment of animal husbandry. Water Sci. Technol. 2011, 63, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, H.; Oturan, N.; Wang, Y.; Chen, L.; Oturan, M.A. Application of response surface methodology to the removal of the antibiotic tetracycline by electrochemical process using carbon-felt cathode and DSA (Ti/RuO2–IrO2) anode. Chemosphere 2012, 87, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Oturan, N.; Wu, J.; Zhang, H.; Sharma, V.K.; Oturan, M.A. Electrocatalytic destruction of the antibiotic tetracycline in aqueous medium by electrochemical advanced oxidation processes: Effect of electrode materials. Appl. Catal. B Environ. 2013, 140, 92–97. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Wang, H.; Song, X.; Wang, T.; He, B.; Liang, X.; Ngo, H.H. An electrocatalytic membrane reactor with self-cleaning function for industrial wastewater treatment. Angew. Chem. Int. Ed. 2011, 50, 2148–2150. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Li, J.; He, B.; Wang, T.; Liao, S. Novel functionalized nano-TiO2 loading electrocatalytic membrane for oily wastewater treatment. Environ. Sci. Technol. 2012, 46, 6815–6821. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, Q.; Li, J.; Wang, T. Phenolic wastewater treatment by an electrocatalytic membrane reactor. Catal. Today 2014, 236, 121–126. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Zhou, Z.; Wang, T.; Ong, C.N.; Vecitis, C.D. Degradation of the common aqueous antibiotic tetracycline using a carbon nanotube electrochemical filter. Environ. Sci. Technol. 2015, 49, 7974–7980. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tian, F.; Liu, C.; Wang, Z.; Du, Z.; Li, R.; Zhang, L. One-step synthesis of nanohybrid carbon dots and TiO2 composites with enhanced ultraviolet light active photocatalysis. RSC Adv. 2015, 5, 8389–8396. [Google Scholar] [CrossRef]
- Ren, W.; Ai, Z.; Jia, F.; Zhang, L.; Fan, X.; Zou, Z. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Wang, C.; Meng, F.; Wang, T.; Ma, T.; Qiu, J. Monolithic coal-based carbon counter electrodes for highly efficient dye-sensitized solar cells. Carbon 2014, 67, 465–474. [Google Scholar] [CrossRef]
- Lei, Y.P.; Wang, Y.D.; Song, Y.C. Atmosphere influence in the pyrolysis of poly [(alkylamino) borazine] for the production of bn fibers. Ceram. Int. 2013, 39, 6847–6851. [Google Scholar] [CrossRef]
- Lei, Y.P.; Wang, Y.D.; Song, Y.C. Effect of temperature on the composition and properties of poly [(alkylamino) borazine] precursor to boron nitride. J. Macromol. Sci. B 2013, 52, 1427–1437. [Google Scholar] [CrossRef]
- Lei, Y.P.; Wang, Y.D.; Song, Y.C. Boron nitride by pyrolysis of the melt-processable poly [tris (methylamino) borane]: Structure, composition and oxidation resistance. Ceram. Int. 2012, 38, 271–276. [Google Scholar] [CrossRef]
- Bin, D.; Wang, H.; Li, J.; Wang, H.; Yin, Z.; Kang, J.; He, B.; Li, Z. Controllable oxidation of glucose to gluconic acid and glucaric acid using an electrocatalytic reactor. Electrochim. Acta 2014, 130, 170–178. [Google Scholar] [CrossRef]










| Parameters | Value |
|---|---|
| Thickness, mm | 5 |
| Area, cm2 | 9 |
| Average pore size, μm | 0.41 |
| Porosity, % | 41.17 |
| Electrical resistivity, mΩ·m | 4.5 |
| Membrane | Element/wt % | ||
|---|---|---|---|
| C | O | Ti | |
| Original carbon membrane | 88.64 | 11.36 | 0 |
| Nano-TiO2/carbon membrane surface | 72.64 | 14.91 | 12.45 |
| Nano-TiO2/carbon membrane cross-section | 82.44 | 12.32 | 5.24 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhu, M.; Wang, Z.; Wang, H.; Deng, C.; Li, K. Effective Degradation of Aqueous Tetracycline Using a Nano-TiO2/Carbon Electrocatalytic Membrane. Materials 2016, 9, 364. https://doi.org/10.3390/ma9050364
Liu Z, Zhu M, Wang Z, Wang H, Deng C, Li K. Effective Degradation of Aqueous Tetracycline Using a Nano-TiO2/Carbon Electrocatalytic Membrane. Materials. 2016; 9(5):364. https://doi.org/10.3390/ma9050364
Chicago/Turabian StyleLiu, Zhimeng, Mengfu Zhu, Zheng Wang, Hong Wang, Cheng Deng, and Kui Li. 2016. "Effective Degradation of Aqueous Tetracycline Using a Nano-TiO2/Carbon Electrocatalytic Membrane" Materials 9, no. 5: 364. https://doi.org/10.3390/ma9050364
APA StyleLiu, Z., Zhu, M., Wang, Z., Wang, H., Deng, C., & Li, K. (2016). Effective Degradation of Aqueous Tetracycline Using a Nano-TiO2/Carbon Electrocatalytic Membrane. Materials, 9(5), 364. https://doi.org/10.3390/ma9050364