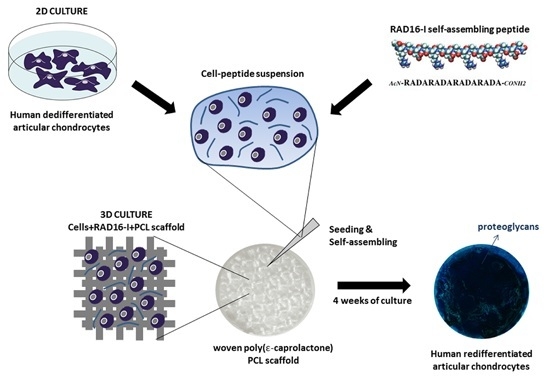
Dedifferentiated Human Articular Chondrocytes Redifferentiate to a Cartilage-Like Tissue Phenotype in a Poly(ε-Caprolactone)/Self-Assembling Peptide Composite Scaffold
Abstract
:1. Introduction
2. Results
2.1. PCL/RAD Composite Development
2.2. Chondrocyte Viability during Culture in 3D-Scaffolds
2.3. Chondrogenic Differentiation of Chondrocytes Seeded in 3D-Scaffolds
2.4. SEM Characterization of AC Constructs
2.5. Mechanical Testing
3. Discussion
4. Materials and Methods
4.1. Scaffold Production
4.2. Contact Angle Measurements
4.3. Scanning Electron Microscopy (SEM)
4.4. Scaffold Surface Morphology Evaluation
4.5. 2D Culture of Human Articular Chondrocytes (AC)
4.6. 3D Cell Cultures Techniques
4.7. MTT Assay
4.8. Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
4.9. Western Blot
4.10. Toluidine Blue Staining
4.11. Mechanical Characterization
4.12. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional; |
| PCL | Poly (ε-caprolactone); |
| RAD | RAD16-I peptide; |
| ECM | Extracellular matrix; |
| CTE | Cartilage tissue engineering; |
| FDA | Food and Drug Administration; |
| PEG | Polyethylene glycol; |
| IPN | Interpenetrating network; |
| SEM | scanning electron microscope; |
| AC | Articular chondrocytes; |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; |
| TGF β1 | transforming growth factor-β1; |
| AA2P | L-Ascorbic Acid 2-phosphate; |
| COL1 | Collagen type I; |
| COL2 | Collagen type II; |
| COL10 | Collagen type X; |
| ACAN | Aggrecan; |
| RPL22 | Ribosomal protein L22; |
| qRT-PCR | quantitative Reverse Transcription Polymerase Chain Reaction; |
| GAGs | glycosaminoglycans; |
| PFA | Paraformaldehyde; |
| DMA | Dynamic Mechanical Analysis; |
| G’ | storage modulus; |
| G’’ | loss modulus; |
| G* | complex modulus; |
| PBST | phosphate buffer saline with Tween-20 |
References
- Hunziker, E.B. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr. Cartil. 2002, 10, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.; Jiang, C. Repair of articular cartilage defects: Review and perspectives. J. Formos. Med. Assoc. 2009, 108, 87–101. [Google Scholar] [CrossRef]
- Chung, C.; Burdick, J. Engineering Cartilage Tissue. Adv. Drug Deliv. Rev. 2008, 60, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Kock, L.; Van Donkelaar, C.C.; Ito, K. Tissue engineering of functional articular cartilage: The current status. Cell Tissue Res. 2012, 347, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Hunziker, E.B.; Lippuner, K.; Keel, M.J.B.; Shintani, N. An educational review of cartilage repair: Precepts & practice—Myths & misconceptions—Progress & prospects. Osteoarthr. Cartil. 2014, 23, 334–350. [Google Scholar] [PubMed]
- Johnstone, B.; Alini, M.; Cucchiarini, M.; Dodge, G.R.; Eglin, D.; Guilak, F. Tissue engineering for articular cartilage repair—The state of the art. Eur. Cell Mater. 2013, 25, 248–267. [Google Scholar] [PubMed]
- Asnaghi, M.A.; Candiani, G.; Farè, S.; Fiore, G.B.; Petrini, P.; Raimondi, M.T. Trends in biomedical engineering: Focus on Regenerative Medicine. J. Appl. Biomater. Biomech. 2011, 9, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Castells-Sala, C.; Alemany-Ribes, M.; Fernández-Muiños, T.; Recha-Sancho, L.; López-Chicón, P.; Aloy-Reverté, C.; Caballero-Camino, J.; Márquez-Gil, A.; Semino, C.E. Current Applications of Tissue Engineering in Biomedicine. J. Biochips Tissue Chips 2013, S2, 1–14. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Madry, H.; Guilak, F.; Saris, D.B.; Stoddart, M.J.; Koon Wong, M. A vision on the future of articular cartilage repair. Eur. Cell Mater. 2014, 27, 12–16. [Google Scholar] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Butler, D.L.; Goldstein, S.A.; Baaijens, F.P.T. Biomechanics and mechanobiology in functional tissue engineering. J. Biomech. 2014, 47, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.M.; Fisher, J.P. Chondrocyte signaling and artificial matrices for articular cartilage engineering. Adv. Exp. Med. Biol. 2006, 585, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Moutos, F.T.; Guilak, F. Composite scaffolds for cartilage tissue engineering. Biorheology 2008, 45, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Moutos, F.T.; Freed, L.E.; Guilak, F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat. Mater. 2007, 6, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Li, S.; Hutmacher, D.W.; Coudane, J.; Vert, M. Degradation characteristics of poly(E-caprolactone)-based copolymers and blends. J. Appl. Polym. Sci. 2006, 102, 1681–1687. [Google Scholar] [CrossRef]
- Rohner, D.; Hutmacher, D.W.; Cheng, T.K.; Oberholzer, M.; Hammer, B. In vivo efficacy of bone-marrow-coated polycaprolactone scaffolds for the reconstruction of orbital defects in the pig. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 66, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003, 21, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Semino, C.E. Self-assembling Peptides: From Bio-inspired Materials to Bone Regeneration. J. Dent. Res. 2008, 87, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Matson, J.B.; Stupp, S.I. Self-assembling peptide scaffolds for regenerative medicine. Chem. Commun. 2012, 48, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Semino, C.E. Can We Build Artificial Stem Cell Compartments? J. Biomed. Biotechnol. 2003, 2003, 164–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garreta, E.; Genové, E.; Borrós, S.; Semino, C.E. Osteogenic Differentiation of Mouse Embryonic Stem Cells and Mouse Embryonic Fibroblasts in a Three-Dimensional. Tissue Eng. Part A 2006, 12, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Marí-Buyé, N.; Muiños, T.F.; Borrós, S.; Favia, P.; Semino, C.E. Nanometric self-assembling peptide layers maintain adult hepatocyte phenotype in sandwich cultures. J. Nanobiotechnol. 2010, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Semino, C.E.; Kasahara, J.; Hayashi, Y. Entrapment of Migrating Hippocampal Neural Cells in Three-Dimensional Peptide Nanofiber Scaffold. Tissue Eng. 2004, 10, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Sieminski a, L.; Semino, C.E.; Gong, H.; Kamm, R.D. Primary sequence of ionic self-assembling peptide gels affects endothelial cell adhesion and capillary morphogenesis. J. Biomed. Mater. Res. A 2008, 87, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Dégano, I.R.; Quintana, L.; Vilalta, M.; Horna, D.; Rubio, N.; Borrós, S. The effect of self-assembling peptide nanofiber scaffolds on mouse embryonic fibroblast implantation and proliferation. Biomaterials 2009, 30, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Alemany-Ribes, M.; García-Díaz, M.; Busom, M.; Nonell, S.; Semino, C.E. Toward a 3D Cellular Model for Studying in Vitro the Outcome of Photodynamic Treatments: Accounting for the Effects of Tissue Complexity. Tissue Eng. Part A 2013, 19, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Marí-Buyé, N.; Luque, T.; Navajas, D.; Semino, C. Development of a three-dimensional bone-like construct in a soft self-assembling peptide matrix. Tissue Eng. Part A 2013, 19, 870–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genové, E.; Schmitmeier, S.; Sala, A.; Borrós, S.; Bader, A.; Griffith, L.G. Functionalized self-assembling peptide hydrogel enhance maintenance of hepatocyte activity in vitro. J. Cell Mol. Med. 2009, 13, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Muiños, T.; Recha-Sancho, L.; López-Chicón, P.; Castells-Sala, C.; Mata, A.; Semino, C.E. Bimolecular based heparin and self-assembling hydrogel for tissue engineering applications. Acta Biomater. 2015, 16, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Genove, E.; Shen, C.; Zhang, S.; Semino, C.E. The effect of functionalized self-assembling peptide scaffolds on human aortic endothelial cell function. Biomaterials 2005, 26, 3341–3351. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.E.; Engelmayr, G.C.; Borenstein, J.T.; Moutos, F.T.; Guilak, F. Advanced Material Strategies for Tissue Engineering Scaffolds. Adv. Mater. 2009, 21, 3410–3418. [Google Scholar] [CrossRef] [PubMed]
- Valonen, P.K.; Moutos, F.T.; Kusanagi, A.; Moretti, M.G.; Diekman, B.O.; Welter, J.F. In vitro generation of mechanically functional cartilage grafts based on adult human stem cells and 3D-woven poly(ε-caprolactone) scaffolds. Biomaterials 2010, 31, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Moutos, F.T.; Guilak, F. Functional properties of cell-seeded three-dimensionally woven poly(epsilon-caprolactone) scaffolds for cartilage tissue engineering. Tissue Eng. Part A 2010, 16, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Liao, I.C.; Moutos, F.T.; Estes, B.T.; Zhao, X.; Guilak, F. Composite three-dimensional woven scaffolds with interpenetrating network hydrogels to create functional synthetic articular cartilage. Adv. Funct. Mater. 2013, 23, 5833–5839. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Muiños, T.; Suárez-Muñoz, M.; Sanmartí-Espinal, M.; Semino, C.E. Matrix dimensions, stiffness, and structural properties modulate spontaneous chondrogenic commitment of mouse embryonic fibroblasts. Tissue Eng. Part A 2014, 20, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Quintana, L.; Muiños, T.F.; Genove, E.; Del Mar Olmos, M.; Borrós, S.; Semino, C.E. Early tissue patterning recreated by mouse embryonic fibroblasts in a three-dimensional environment. Tissue Eng. Part A 2009, 15, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, B.M.; Reiche, S.; Marí-Buyé, N.; Castells-Sala, C.; Meisel, J.; Semino, C.E. Chondrogenic potential of human dermal fibroblasts in contractile solft self-assembling peptide hydrogel. J. Tissue Eng. Regen. Med. 2013. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Peeters-Joris, C.; Vaes, G. Production of collagens, collagenase and collagenase inhibitor during the dedifferentiation of articular chondrocytes by serial subcultures. Biochim. Biophys. Acta 1990, 1051, 266–275. [Google Scholar] [CrossRef]
- Darling, E.M.; Athanasiou, K.A. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 2005, 23, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Shin, H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Girotto, D.; Urbani, S.; Brun, P.; Renier, D.; Barbucci, R.; Abatangelo, G. Tissue-specific gene expression in chondrocytes grown on three-dimensional hyaluronic acid scaffolds. Biomaterials 2003, 24, 3265–3275. [Google Scholar] [CrossRef]
- Miot, S.; Woodfield, T.; Daniels, A.U.; Suetterlin, R.; Peterschmitt, I.; Heberer, M. Effects of scaffold composition and architecture on human nasal chondrocyte redifferentiation and cartilaginous matrix deposition. Biomaterials 2005, 26, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, S.E.; Kang, S.S.; Kim, Y.H.; Tae, G. The use of de-differentiated chondrocytes delivered by a heparin-based hydrogel to regenerate cartilage in partial-thickness defects. Biomaterials 2011, 32, 7883–7896. [Google Scholar] [CrossRef] [PubMed]
- Grad, S.; Gogolewski, S. Effects of Simple and Complex Motion Patterns on Gene Expression of Chondrocytes Seeded in 3D Scaffolds. Tissue Eng. 2006, 12, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Recha-Sancho, L.; Semino, C.E. Heparin based self-assembling peptide scaffold re-establish chondrogenic phenotype of expanded de-differentiated human chondrocytes. J. Biomed. Mater. Res. Part A 2016, 104, 1694–1706. [Google Scholar] [CrossRef] [PubMed]
- Cals, F.L.J.; Hellingman, C.A.; Koevoet, W.; Baatenburg de Jong, R.J. Effects of transforming growth factor-β subtypes on in vitro cartilage production and mineralization of human bone marrow stromal-derived mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2012, 6, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Giralt, N.; Izquierdo, R.; Nogués, X.; Perez-Olmedilla, M.; Benito, P.; Checa, M.A.; Suay, J.; Caceres, E.; Monllau, J.C. A porous PCL scaffold promotes the human chondrocytes redifferentiation and hyaline-specific extracellular matrix protein synthesis. J. Biomed. Mater. Res. A 2008, 85, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc. Natl. Acad. Sci. USA 2002, 99, 9996–10001. [Google Scholar] [CrossRef] [PubMed]
- Moutos, F.T.; Estes, B.T.; Guilak, F. Multifunctional Hybrid Three-dimensionally Woven Scaffolds for Cartilage Tissue Engineering. Macromol. Biosci. 2010, 10, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Uygun, B.E.; Geerts, S.; Ozer, S.; Scalf, M.; Gilpin, S.E. Proteomic analysis of naturally-sourced biological scaffolds. Biomaterials 2016, 75, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Darnell, M.; Sun, J.-Y.; Mehta, M.; Johnson, C.; Arany, P.; Suo, Z. Performance and Biocompatibility of Extremely Tough Alginate/Polyacrylamide Hydrogels. Biomaterials 2013, 34, 8042–8048. [Google Scholar] [CrossRef] [PubMed]
- Baugé, C.; Girard, N.; Lhuissier, E.; Bazille, C.; Boumediene, K. Regulation and Role of TGFβ Signaling P athway in Aging and Osteoarthritis Joints. Aging Dis. 2014, 5, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Bobick, B.E.; Chen, F.H.; Le, A.M.; Tuan, R.S. Regulation of the chondrogenic phenotype in culture. Birth Defects Res. Part C Embryo Today Rev. 2009, 87, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Sieminski, A.L.; Was, A.S.; Kim, G.; Gong, H.; Kamm, R.D. The Stiffness of Three-dimensional Ionic Self-assembling Peptide Gels Affects the Extent of Capillary-like Network Formation. Cell Biochem. Biophys. 2007, 49, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.M.; Raimondi, M.T.; Pietrabissa, R. Controlling Self-Renewal and Differentiation of Stem Cells via Mechanical Cues. BioMed Res. Int. 2012. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.M.; Raimondi, M.T.; Credi, C.; De Marco, C.; Turri, S.; Cerullo, G. Interactions between structural and chemical biomimetism in synthetic stem cell niches. Biomed. Mater. 2015, 10, 015012. [Google Scholar] [CrossRef] [PubMed]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Recha-Sancho, L.; Moutos, F.T.; Abellà, J.; Guilak, F.; Semino, C.E. Dedifferentiated Human Articular Chondrocytes Redifferentiate to a Cartilage-Like Tissue Phenotype in a Poly(ε-Caprolactone)/Self-Assembling Peptide Composite Scaffold. Materials 2016, 9, 472. https://doi.org/10.3390/ma9060472
Recha-Sancho L, Moutos FT, Abellà J, Guilak F, Semino CE. Dedifferentiated Human Articular Chondrocytes Redifferentiate to a Cartilage-Like Tissue Phenotype in a Poly(ε-Caprolactone)/Self-Assembling Peptide Composite Scaffold. Materials. 2016; 9(6):472. https://doi.org/10.3390/ma9060472
Chicago/Turabian StyleRecha-Sancho, Lourdes, Franklin T. Moutos, Jordi Abellà, Farshid Guilak, and Carlos E. Semino. 2016. "Dedifferentiated Human Articular Chondrocytes Redifferentiate to a Cartilage-Like Tissue Phenotype in a Poly(ε-Caprolactone)/Self-Assembling Peptide Composite Scaffold" Materials 9, no. 6: 472. https://doi.org/10.3390/ma9060472
APA StyleRecha-Sancho, L., Moutos, F. T., Abellà, J., Guilak, F., & Semino, C. E. (2016). Dedifferentiated Human Articular Chondrocytes Redifferentiate to a Cartilage-Like Tissue Phenotype in a Poly(ε-Caprolactone)/Self-Assembling Peptide Composite Scaffold. Materials, 9(6), 472. https://doi.org/10.3390/ma9060472