Oil-in-Water Self-Assembled Synthesis of Ag@AgCl Nano-Particles on Flower-like Bi2O2CO3 with Enhanced Visible-Light-Driven Photocatalytic Activity
Abstract
:1. Introduction
2. Experimental
2.1. Photocatalyst Synthesis
2.2. Photocatalyst Characterization
2.3. Photocatalytic Activity
3. Result and Discussion
3.1. Catalyst Characterization
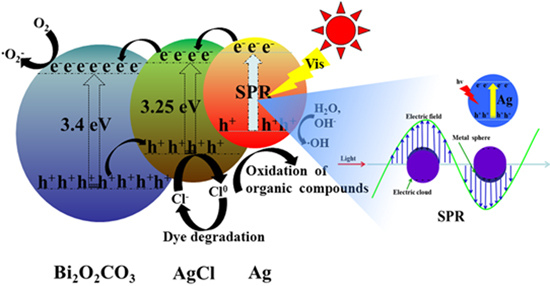
3.2. Photocatalytic Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, J.W.; Zou, Z.G.; Ye, J.H. Efficient photocatalysis on BaBiO3 driven by visible light. J. Phys. Chem. C 2007, 111, 12779–12785. [Google Scholar] [CrossRef]
- Zhang, L.; Hashimoto, Y.; Taishi, T.; Nakamur, I.; Ni, Q.Q. Fabrication of flower-shaped Bi2O3 superstructure by a facile template-free process. Appl. Surf. Sci. 2011, 257, 6577–6582. [Google Scholar] [CrossRef]
- Dumrongrojthanath, P.; Thongtem, T.; Phuruangrat, A.; Thongtem, S. Hydrothermal synthesis of Bi2WO6 hierarchical flowers with their photonic and photocatalytic properties. Superlattice Microstruct. 2013, 54, 71–77. [Google Scholar] [CrossRef]
- Peng, S.J.; Li, L.L.; Zhu, P.N.; Wu, Y.Z.; Srinivasan, M.; Mhaisalkar, S.G.; Ramakrishna, S.; Yan, Q.Y. Controlled synthesis of BiOCl hierarchical self-assemblies with highly efficient photocatalytic properties. Chem. Asian J. 2013, 8, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, F.J.; Lin, J.; Chen, D.F.; Gao, J.M.; Huang, Z.X.; Ding, X.X.; Tang, C.C. Self-assembled 3-D architectures of BiOBr as a visible lightdriven photocatalyst. Chem. Mater. 2008, 20, 2937–2941. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, G.; So, M.H.; Wu, J.L.; Lu, Z.; Che, C.M.; Sun, H.Z. Bismuth subcarbonate nanoparticles fabricated by water-in-oil microemulsionassisted hydrothermal process exhibit anti-Helicobacter pyloriproperties. Mater. Res. Bull. 2010, 45, 654–658. [Google Scholar] [CrossRef]
- Hu, R.P.; Xiao, X.; Tu, S.H.; Zuo, X.X.; Nan, J.M. Synthesis of flower-like heterostructured β-Bi2O3/Bi2O2CO3 microspheres using Bi2O2CO3 self-sacrifice precursor and its visible-light-induced photocatalytic degradation of o-phenylphenol. Appl. Catal. B 2015, 163, 510–519. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.J.; Fu, M.; Ho, W.K.; Lee, S.C.; Wu, Z.B. Novel in situ N-doped (BiO)2CO3 hierarchical microspheres self-assembled by nanosheets as efficient and durable visible light driven photocatalyst. Langmuir 2012, 28, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.Y.; Zai, J.T.; Xu, M.; Zou, Q.; Su, Y.Z.; Wang, K.X.; Qian, X.F. Hierarchical Bi2O2CO3 microspheres with improved visible-light-driven photocatalytic activity. CrystEngComm 2011, 13, 4010–4017. [Google Scholar] [CrossRef]
- Madhusudan, P.; Yu, J.G.; Wang, W.G.; Cheng, B.; Liu, G. Facile synthesis of novel hierarchical grapheme-Bi2O2CO3 composites with enhanced photocatalytic performance under visible light. Dalton Trans. 2012, 41, 14345–14353. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, R.; Yin, S.F.; Luo, S.L.; Au, C.T. Flower-like Bi2O2CO3: Facile synthesis and their photocatalytic application in treatment of dyecontaining wastewater. Chem. Eng. J. 2012, 193–194, 123–130. [Google Scholar] [CrossRef]
- Cao, X.F.; Zhang, L.; Chen, X.T.; Xue, Z.L. Persimmon-like (BiO)2CO3 microstructures: Hydrothermal preparation, photocatalytic properties and their conversion into Bi2S3. CrystEngComm 2011, 13, 1939–1945. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Z.Y.; Huang, B.B.; Yang, K.S.; Zhang, X.Y.; Qin, X.Y.; Dai, Y. Preparation, electronic structure, and photocatalytic properties of Bi2O2CO3 nanosheet. Appl. Surf. Sci. 2010, 257, 172–175. [Google Scholar] [CrossRef]
- Zheng, Y.; Duan, F.; Chen, M.Q.; Xie, Y. Synthetic Bi2O2CO3 nanostructures: Novel photocatalyst with controlled special surface exposed. J. Mol. Catal. A Chem. 2010, 317, 34–40. [Google Scholar] [CrossRef]
- Cheng, H.F.; Huang, B.B.; Yang, K.S.; Wang, Z.Y.; Qin, X.Y.; Zhang, X.Y.; Dai, Y. Facile template-free synthesis of Bi2O2CO3 hierarchical microflowers and their associated photocatalytic activity. Chemphyschem 2010, 11, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.H.; Zhang, X.Y.; Li, G.W.; Cao, Y.T.; Shao, Y.; Li, D.Z. Highly efficient Bi2O2CO3/BiOCl photocatalyst based on heterojunction with enhanced dye-sensitization under visible light. Appl. Catal. B 2016, 187, 301–309. [Google Scholar] [CrossRef]
- Kowalska, E.; Remita, H.; Colbeau-Justin, C.; Hupka, J.; Belloni, J. Modification of titanium dioxide with platinum ions and clusters: Application in photocatalysis. J. Phys. Chem. C 2008, 112, 1124–1131. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Yun, G.X.; Bai, Y.; An, N.; Lian, J.H.; Huang, H.H.; Su, B.T. Photodegradation of rhodamine B with MoS2/Bi2O2CO3 composites under UV light irradiation. Appl. Surf. Sci. 2014, 313, 537–544. [Google Scholar] [CrossRef]
- Tian, N.; Huang, H.W.; Guo, Y.X.; He, Y.; Zhang, Y.H. A g-C3N4/Bi2O2CO3 composite with high visible-light-driven photocatalytic activity for rhodamine B degradation. Appl. Surf. Sci. 2014, 322, 249–254. [Google Scholar] [CrossRef]
- Hu, D.D.; Zhang, K.Y.; Yang, Q.; Wang, M.J.; Xi, Y.; Hu, C.G. Super-high photocatalytic activity of Fe2O3 nanoparticles anchored on Bi2O2CO3 nanosheets with exposed {0 0 1} active facets. Appl. Surf. Sci. 2014, 316, 93–101. [Google Scholar] [CrossRef]
- Jin, L.; Zhu, G.Q.; Hojamberdiev, M.; Luo, X.C.; Tan, C.W.; Peng, J.H.; Wei, X.M.; Li, J.P.; Liu, P. A Plasmonic Ag–AgBr/Bi2O2CO3 Composite Photocatalyst with Enhanced Visible-Light Photocatalytic Activity. Ind. Eng. Chem. Res. 2014, 35, 13718–13727. [Google Scholar] [CrossRef]
- Zhang, L.S.; Wong, K.H.; Chen, Z.G.; Yu, J.C.; Zhao, J.C.; Hu, C.; Chang, C.Y.; Wong, P.K. AgBr-Ag-Bi2WO6 nanojunction system: A novel and efficient photocatalyst with double visible-light active components. Appl. Catal. A 2009, 363, 221–229. [Google Scholar] [CrossRef]
- Hu, X.X.; Hu, C.; Peng, T.W.; Zhou, X.F.; Qu, J.H. Plasmon-induced inactivation of enteric pathogenic microorganisms with Ag-AgI/Al2O3 under visible-light irradiation. Environ. Sci. Technol. 2010, 44, 7058–7062. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Li, Q.Y.; Zhou, Y.; Sun, Y.J.; Zhang, H.D.; Wu, Z.B. In situ decoration of plasmonic Ag nanocrystals on the surface of (BiO)2CO3 hierarchical microspheres for enhanced visible light photocatalysis. Dalton Trans. 2014, 43, 9468–9480. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.J.; Li, L.L.; Tan, H.T.; Wu, Y.Z.; Cai, R.; Yu, H.; Huang, X.; Zhu, P.N.; Ramakrishna, S.; Srinivasana, M.; et al. Monodispersed Ag nanoparticles loaded on the PVP-assisted synthetic Bi2O2CO3 microspheres with enhanced photocatalytic and supercapacitive performances. J. Mater. Chem. A 2013, 1, 7630–7638. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.G.; Ho, W.K.; Jiang, Z.T.; Zhang, L.Z. Effects of F-doping on the photocatalytic activity and microstructures of nanocrystalline TiO2 powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Zheng, L.H.; Chen, Y.T.; Fan, J.F.; Huang, H.H.; Su, B.T. Synthesis and characterization of novel PPy/Bi2O2CO3 composite with improved photocatalytic activity for degradation of Rhodamine-B. J. Alloys Compd. 2015, 637, 127–132. [Google Scholar] [CrossRef]
- Huang, H.W.; Wang, S.B.; Tian, N.; Zhang, Y.H. One-step hydrothermal preparation strategy for layered BiIO4/Bi2WO6 heterojunctions with enhanced visible light photocatalytic activities. RSC Adv. 2014, 4, 5561–5567. [Google Scholar] [CrossRef]
- He, Y.M.; Cai, J.; Zhang, L.H.; Wang, X.X.; Lin, H.J.; Teng, B.T.; Zhao, L.H.; Weng, W.Z.; Wan, H.L.; Fan, M.H. Comparing two new composite photocatalysts, t-LaVO4/g-C3N4 and m-LaVO4/g-C3N4, for their structures and performances. Ind. Eng. Chem. Res. 2014, 53, 5905–5915. [Google Scholar] [CrossRef]
- Huang, H.W.; Yao, J.Y.; Lin, Z.S.; Wang, X.Y.; He, R.; Yao, W.J.; Zhai, N.X.; Chen, C.T. NaSr3Be3B3O9F4: A promising deep-ultraviolet nonlinear optical material resulting from the cooperative alignment of the [Be3B3O12F]10− anionic group. Angew. Chem. Int. Ed. 2011, 50, 9141–9144. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.G.; Yu, H.G.; Cheng, B.; Zhao, X.J.; Yu, J.C.; Ho, W.K. The effects of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Ao, Y.H.; Xu, L.Y.; Wang, P.F.; Wang, C.; Hou, J.; Qian, J. Preparation of CdS nanoparticle loadedflower-like Bi2O2CO3 heterojunction photocatalysts with enhanced visible light photocatalytic activity. Dalton Trans. 2015, 44, 11321–11330. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.D.; Dai, W.X.; Tian, Q.F.; Li, Z.H.; Xie, L.Y.; Wang, J.X.; Liu, P. Photocatalytic degradation of RhB over TiO2 bilayer films: Effect of defects and their location. Langmuir 2010, 26, 9686–9694. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.F.; Han, Y.D.; Gao, S.; Zhang, X.L. Surface modification of titania-coated cobalt ferrite magnetic photocatalyst by cold plasma. Surf. Coat. Technol. 2013, 228, 516–519. [Google Scholar] [CrossRef]
- Li, W.J.; Li, D.Z.; Wang, J.X.; Shao, Y.; You, J.M.; Teng, F. Exploration of the active species in the photocatalytic degradation of methyl orange under UV light irradiation. J. Mol. Catal. A Chem. 2013, 380, 10–17. [Google Scholar] [CrossRef]
- Cao, J.; Xu, B.Y.; Lin, H.L.; Luo, B.D.; Chen, S.F. Chemical etching preparation of BiOI/BiOBr heterostructures with enhanced photocatalytic properties for organic dye removal. Chem. Eng. J. 2012, 185–186, 91–99. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Long, M.C.; Cai, W.M.; Cai, J. Synthesis and photocatalytic performance of the efficient visible light photocatalyst Ag-AgCl/BiVO4. J. Mol. Catal. A Chem. 2012, 353–354, 22–28. [Google Scholar] [CrossRef]
- Xu, Y.G.; Xu, H.; Yan, J.; Li, H.M.; Huang, L.Y.; Xi, J.X.; Yin, S.; Shu, H.M. A plasmonic photocatalyst of Ag/AgBr nanoparticles coupled with g-C3N4 with enhanced visible-light photocatalytic ability. Colloids Surf. A 2013, 436, 474–483. [Google Scholar] [CrossRef]
- Huang, H.W.; Li, X.W.; Wang, J.J.; Dong, F.; Chu, P.K.; Zhang, T.R.; Zhang, Y.H. Anionic group self-doping as a promising strategy: Band-gap engineering and multi-functional applications of high-performance CO32−-doped Bi2O2CO3. ACS Catal. 2015, 5, 4094–4103. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.Y.; Hu, S.Z.; Lu, G.; Bai, J.; Kang, X.X.; Liu, D.; Gui, J.Z. Synthesis and properties of visible light responsive g-C3N4/Bi2O2CO3 layered heterojunction nanocomposites. RSC Adv. 2015, 5, 42736–42743. [Google Scholar] [CrossRef]
- He, B.L.; Dong, B.; Li, H.L. Preparation and electrochemical properties of Ag-modified TiO2 nanotube anode material for lithium-ion battery. Electrochem. Commun. 2007, 9, 425–430. [Google Scholar] [CrossRef]
- Huang, Q.W.; Tian, S.Q.; Zeng, D.W.; Wang, X.X.; Song, W.L.; Li, Y.Y.; Xiao, W.; Xie, C.S. Enhanced photocatalytic activity of chemically bonded TiO2/graphene composites based on the effective interfacial charge transfer through the C-Ti bond. ACS Catal. 2013, 3, 1477–1485. [Google Scholar] [CrossRef]
- Liang, Y.H.; Lin, S.L.; Hu, J.S.; Liu, L.; McEvoy, J.G.; Cui, W.Q. Facile hydrothermal synthesis of nanocomposite Ag@AgCl/K2Ti4O9 and photocatalytic degradation under visible light irradiation. J. Mol. Catal. A Chem. 2014, 383–384, 231–238. [Google Scholar] [CrossRef]
- Chen, L.; Yin, S.F.; Luo, S.L.; Huang, R.; Zhang, Q.; Hong, T.; Au, P.C.T. Bi2O2CO3/BiOI photocatalysts with heterojunctions highly efficient for visible-light treatment of dye-containing wastewater. Ind. Eng. Chem. Res. 2012, 51, 6760–6768. [Google Scholar] [CrossRef]
- Hu, C.; Lan, Y.Q.; Qu, J.H.; Hu, X.X.; Wang, A.M. Ag/AgBr/TiO2 visible light photocatalyst for destruction of azodyes and bacteria. J. Phys. Chem. B 2006, 110, 4066–4072. [Google Scholar] [CrossRef] [PubMed]















| Photocatalysts | Bi2O2CO3 | Ag@AgCl (5 wt %)/ Bi2O2CO3 | Ag@AgCl (20 wt %)/ Bi2O2CO3 | Ag@AgCl (25 wt %)/ Bi2O2CO3 |
|---|---|---|---|---|
| Surface area (m2·g−1) | 13.61 | 14.23 | 22.07 | 24.23 |
| Major Element | Ag | Cl | Bi | C | O |
|---|---|---|---|---|---|
| Ag@AgCl (10 wt %)/Bi2O2CO3 (content, mass %) | 7.55 | 1.68 | 67.28 | 2.08 | 16.81 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Liu, L.; Liang, Y.; Cui, W.; Zhang, Z. Oil-in-Water Self-Assembled Synthesis of Ag@AgCl Nano-Particles on Flower-like Bi2O2CO3 with Enhanced Visible-Light-Driven Photocatalytic Activity. Materials 2016, 9, 486. https://doi.org/10.3390/ma9060486
Lin S, Liu L, Liang Y, Cui W, Zhang Z. Oil-in-Water Self-Assembled Synthesis of Ag@AgCl Nano-Particles on Flower-like Bi2O2CO3 with Enhanced Visible-Light-Driven Photocatalytic Activity. Materials. 2016; 9(6):486. https://doi.org/10.3390/ma9060486
Chicago/Turabian StyleLin, Shuanglong, Li Liu, Yinghua Liang, Wenquan Cui, and Zisheng Zhang. 2016. "Oil-in-Water Self-Assembled Synthesis of Ag@AgCl Nano-Particles on Flower-like Bi2O2CO3 with Enhanced Visible-Light-Driven Photocatalytic Activity" Materials 9, no. 6: 486. https://doi.org/10.3390/ma9060486
APA StyleLin, S., Liu, L., Liang, Y., Cui, W., & Zhang, Z. (2016). Oil-in-Water Self-Assembled Synthesis of Ag@AgCl Nano-Particles on Flower-like Bi2O2CO3 with Enhanced Visible-Light-Driven Photocatalytic Activity. Materials, 9(6), 486. https://doi.org/10.3390/ma9060486