Physico-Chemical Alternatives in Lignocellulosic Materials in Relation to the Kind of Component for Fermenting Purposes
Abstract
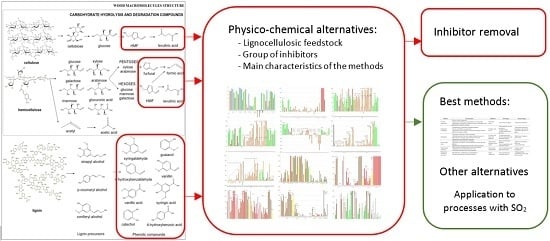
:1. Introduction
2. Inhibitors in Lignocellulosic Materials
2.1. Furan Inhibitors
2.2. Weak Acids
2.3. Phenolic Compounds
2.4. Other Inhibitors
2.5. Synergistic Effects
3. Physico-Chemical Detoxification Processes
3.1. Evaporation
 eucalyptus wood hydrolysates, whereas xylose and arabinose are between 51% and 62% in the case of eucalyptus hemicellulosic hydrolysates [62] and from 81% to 99% in the case of Eucalyptus grandis [50], pointing out the importance of the optimisation of this method for hydrolysates with more pentose sugar content. On the other hand, when vacuum evaporation for
eucalyptus wood hydrolysates, whereas xylose and arabinose are between 51% and 62% in the case of eucalyptus hemicellulosic hydrolysates [62] and from 81% to 99% in the case of Eucalyptus grandis [50], pointing out the importance of the optimisation of this method for hydrolysates with more pentose sugar content. On the other hand, when vacuum evaporation for  rice straw hydrolysates is used [37,117], a slight increase of the xylose (13% higher) and arabinose (15%–17% higher) is found in relation to glucose.
rice straw hydrolysates is used [37,117], a slight increase of the xylose (13% higher) and arabinose (15%–17% higher) is found in relation to glucose. soybean hulls hydrolysate [116] and
soybean hulls hydrolysate [116] and  olive tree pruning hydrolysates [118]. In both cases, the reason was probably due to the pH of the sample, close to 5.5. Therefore, a previous neutralisation of the liquor is not recommended to remove this kind of pollutant; however, if the valorisation of this compound is the objective, a previous neutralisation is recommended. Regarding HMF, worse results are obtained in all cases. The best result in this case was in the sample of
olive tree pruning hydrolysates [118]. In both cases, the reason was probably due to the pH of the sample, close to 5.5. Therefore, a previous neutralisation of the liquor is not recommended to remove this kind of pollutant; however, if the valorisation of this compound is the objective, a previous neutralisation is recommended. Regarding HMF, worse results are obtained in all cases. The best result in this case was in the sample of  rice straw hydrolysate with no previous neutralisation, giving a detoxification of more than 80% in relation to the final concentration of glucose [37]. Regarding the concentration of phenolics, a final percentage between 62% and 92% in relation to the concentration of glucose is given; therefore, only a maximum evaporation of about 40% is obtained.
rice straw hydrolysate with no previous neutralisation, giving a detoxification of more than 80% in relation to the final concentration of glucose [37]. Regarding the concentration of phenolics, a final percentage between 62% and 92% in relation to the concentration of glucose is given; therefore, only a maximum evaporation of about 40% is obtained.3.2. Liming and Overliming
 olive residues have been detoxified. The best results have been given in the case of levulinic acid for
olive residues have been detoxified. The best results have been given in the case of levulinic acid for  brewery’s spent grain hydrolysates [119]. In this case, Ca(OH)2 at pH 10 and 1 h of process is used. Results close to 50% of acetic acid have been obtained in the case of
brewery’s spent grain hydrolysates [119]. In this case, Ca(OH)2 at pH 10 and 1 h of process is used. Results close to 50% of acetic acid have been obtained in the case of  olive residues liming at pH equal to 5.5 using Ca(OH)2 during 10 min. The same results have been obtained when overliming at pH 10 with Ca(OH)2 or CaO during 10 min followed by a decrease of the pH to 5.5 with H2SO4 is used [118] and for formic acid when overliming with Ca(OH)2 is used with a previous water extraction [97]. The detoxification of weak acids in the rest of the experiments is close to 20%.
olive residues liming at pH equal to 5.5 using Ca(OH)2 during 10 min. The same results have been obtained when overliming at pH 10 with Ca(OH)2 or CaO during 10 min followed by a decrease of the pH to 5.5 with H2SO4 is used [118] and for formic acid when overliming with Ca(OH)2 is used with a previous water extraction [97]. The detoxification of weak acids in the rest of the experiments is close to 20%. olive tree pruning or olive stones,
olive tree pruning or olive stones,  sugarcane bagasse,
sugarcane bagasse,  rice straw, and
rice straw, and  Kappaphycus alvarezii (cottonii), a maximum of 80% detoxification is obtained; however; (ii) when
Kappaphycus alvarezii (cottonii), a maximum of 80% detoxification is obtained; however; (ii) when  brewery’s spent grain hydrolysate or
brewery’s spent grain hydrolysate or  spruce hydrolysate are treated, close to 100% is obtained in both furfural and HMF in most cases [119,120]. In all cases, an increase in time (red arrows in the figure) and pH in the experiments gives better results of both furfural and HMF; however, the increase of temperature does not affect the detoxification process as much. In the results of Millati et al. [120], the use of Ca(OH)2 with a pH close to 12 with a reaction time of more than 20 h is recommended to obtain detoxification results close to 100%. When NaOH or NH4OH is used, instead of Ca(OH)2, maximum percentages of removal between 33% and 43% in the case of furfural and 23% and 47% for HMF are obtained, with the best results, from 40% to 47%, occurring when NH4OH is used [113,118].
spruce hydrolysate are treated, close to 100% is obtained in both furfural and HMF in most cases [119,120]. In all cases, an increase in time (red arrows in the figure) and pH in the experiments gives better results of both furfural and HMF; however, the increase of temperature does not affect the detoxification process as much. In the results of Millati et al. [120], the use of Ca(OH)2 with a pH close to 12 with a reaction time of more than 20 h is recommended to obtain detoxification results close to 100%. When NaOH or NH4OH is used, instead of Ca(OH)2, maximum percentages of removal between 33% and 43% in the case of furfural and 23% and 47% for HMF are obtained, with the best results, from 40% to 47%, occurring when NH4OH is used [113,118]. olive tree residues are treated [118], 41% for
olive tree residues are treated [118], 41% for  sugarcane bagasse [96], and 29% in the case of
sugarcane bagasse [96], and 29% in the case of  spruce hydrolysates [120].
spruce hydrolysates [120]. olive stones as raw material with losses from 76% to 100% of xylose [123], hydrolysate of
olive stones as raw material with losses from 76% to 100% of xylose [123], hydrolysate of  Kappaphycus alvarezii with losses of 86% of glucose and 77% of galactose [77], and in the most aggressive conditions, in the case of
Kappaphycus alvarezii with losses of 86% of glucose and 77% of galactose [77], and in the most aggressive conditions, in the case of  spruce hydrolysate, using a pH value of 12 with Ca(OH)2 and a reaction time of more than 20 h (the same conditions when furans are completely removed). In this case, losses of glucose from 65% to 71% at 60 °C and from 33% to 47% at 25 °C; xylose from 87% to 88% at 60 °C and from 75% to 77% at 25 °C; mannose from 64% to 69% at 60 °C and from 30% to 48% at 25 °C; and galactose from 69% to 71% at 60 °C and from 69% to 86% at 25 °C are obtained [120].
spruce hydrolysate, using a pH value of 12 with Ca(OH)2 and a reaction time of more than 20 h (the same conditions when furans are completely removed). In this case, losses of glucose from 65% to 71% at 60 °C and from 33% to 47% at 25 °C; xylose from 87% to 88% at 60 °C and from 75% to 77% at 25 °C; mannose from 64% to 69% at 60 °C and from 30% to 48% at 25 °C; and galactose from 69% to 71% at 60 °C and from 69% to 86% at 25 °C are obtained [120].3.3. Adsorption
 brewery’s spent grain [119],
brewery’s spent grain [119],  sugarcane bagasse [57],
sugarcane bagasse [57],  hardwood chips [10],
hardwood chips [10],  soybean hulls [116],
soybean hulls [116],  Eucalyptus grandis [62],
Eucalyptus grandis [62],  Kappaphycus alvarezii [77],
Kappaphycus alvarezii [77],  olive tree pruning residue [118], and
olive tree pruning residue [118], and  rape straw [106] hydrolysates. However, the kind of raw material has no influence on the adsorption results.
rape straw [106] hydrolysates. However, the kind of raw material has no influence on the adsorption results. sugarcane bagasse [57] and 42% for
sugarcane bagasse [57] and 42% for  hardwood chips [10], respectively; and the losses of sugars are under 27% of glucose and 43% of arabinose in the case of
hardwood chips [10], respectively; and the losses of sugars are under 27% of glucose and 43% of arabinose in the case of  Eucalyptus grandis [62], 8% for mannose for
Eucalyptus grandis [62], 8% for mannose for  soybean hulls [116], 20% of galactose when
soybean hulls [116], 20% of galactose when  Kappaphycus alvarezii hydrolysates are detoxified [77], and only 8% of xylose in the case of
Kappaphycus alvarezii hydrolysates are detoxified [77], and only 8% of xylose in the case of  soybean hulls [116]. On the other hand, regarding the adsorption of acetic acid, in spite of having a low value, the best results are obtained in the case of using lower pHs in the hydrolysate, from 1.8 to 2.5, according to the results of Villareal et al. [62] and Schirmer-Michel et al. [116]. This behaviour is also shown in the results of HMF and phenolics; however, the losses of sugars in this case are higher [62].
soybean hulls [116]. On the other hand, regarding the adsorption of acetic acid, in spite of having a low value, the best results are obtained in the case of using lower pHs in the hydrolysate, from 1.8 to 2.5, according to the results of Villareal et al. [62] and Schirmer-Michel et al. [116]. This behaviour is also shown in the results of HMF and phenolics; however, the losses of sugars in this case are higher [62].3.4. Ion Exchange Resins
 Picea abies and
Picea abies and  corn stover hydrolysates, respectively. In addition, very good results have been obtained for acetic acid for
corn stover hydrolysates, respectively. In addition, very good results have been obtained for acetic acid for  Eucalyptus grandis [62]. However, low results of acids (acetic and formic) are obtained for
Eucalyptus grandis [62]. However, low results of acids (acetic and formic) are obtained for  brewery’s spent grain hydrolysate [119]. When cationic resins IRN-77 and XAD-X8 (BioRad Laboratories) are used, removals up to only 14% for acetic acid and 23% of formic acid are obtained, while removals close to 100% are obtained in the case of levulinic acid [98,119]. The pH needs to be optimised in all cases, giving better results at lower pHs.
brewery’s spent grain hydrolysate [119]. When cationic resins IRN-77 and XAD-X8 (BioRad Laboratories) are used, removals up to only 14% for acetic acid and 23% of formic acid are obtained, while removals close to 100% are obtained in the case of levulinic acid [98,119]. The pH needs to be optimised in all cases, giving better results at lower pHs. corn stover [130],
corn stover [130],  brewery’s spent grain [119], and
brewery’s spent grain [119], and  Eucalyptus grandis [62] hydrolysates.
Eucalyptus grandis [62] hydrolysates. Eucalyptus grandis at lower pHs (1.8), giving 44% losses of glucose and 29% of arabinose [62].
Eucalyptus grandis at lower pHs (1.8), giving 44% losses of glucose and 29% of arabinose [62].3.5. Liquid–Liquid Extraction
 olive tree pruning residue [118], sugarcane bagasse [131]
olive tree pruning residue [118], sugarcane bagasse [131]  corn stover [63,132], aspen [133],
corn stover [63,132], aspen [133],  wood [134], and
wood [134], and  synthetic [135] hydrolysates. Both ethyl acetate and trialkylamine give the best results for furans and phenolics [63,118,132,133,134], trialkylamine, and trichloroethylene in the case of acids [63,132,134]. Wilson et al. [133] found that ethyl acetate extraction was more effective than roto-evaporation in removing the inhibitors. The roto-evaporation removed furfural and most of the acetic acid but did not reduce lignin-derivative levels. The ethyl acetate extraction removed all the inhibitory compounds, except acetic acid, which was not completely removed by the ethyl acetate extraction process [51,133].
synthetic [135] hydrolysates. Both ethyl acetate and trialkylamine give the best results for furans and phenolics [63,118,132,133,134], trialkylamine, and trichloroethylene in the case of acids [63,132,134]. Wilson et al. [133] found that ethyl acetate extraction was more effective than roto-evaporation in removing the inhibitors. The roto-evaporation removed furfural and most of the acetic acid but did not reduce lignin-derivative levels. The ethyl acetate extraction removed all the inhibitory compounds, except acetic acid, which was not completely removed by the ethyl acetate extraction process [51,133].3.6. Filtration by Membrane Operations
 rice straw [140] and some
rice straw [140] and some  synthetic [52,141] hydrolysates. However, depending on the kind of sugar, high losses can be obtained, from glucose (up to 5%), xylose and arabinose (up to 14%), and mannose and galactose (up to 30%) [17,52,141,142,143,144,145].
synthetic [52,141] hydrolysates. However, depending on the kind of sugar, high losses can be obtained, from glucose (up to 5%), xylose and arabinose (up to 14%), and mannose and galactose (up to 30%) [17,52,141,142,143,144,145].3.7. Combination Processes
 Eucalyptus wood [50,147],
Eucalyptus wood [50,147],  ponderosa pine wood [43], and
ponderosa pine wood [43], and  rice straw [117] hydrolysates have been studied. The best results are obtained for overliming + ethyl acetate extraction + activated charcoal adsorption for phenolics for eucalyptus wood hydrolysates [147] and the use of activated charcoal or diatomaceous earths + anionic resin in the case of furans for eucalyptus wood [50]. In addition, using flocculation + resin-wafer electrodeionisation (RW-EDI), good results in all of the inhibitors have been obtained, with a removal of 60%–74% of furans, 77% acetic acid, and 97% of sulphuric acid when Ponderosa pine wood hydrolysate is used as the raw material [43]. Both processes, flocculation + resin-wafer electrodeionisation, are explained in the following section.
rice straw [117] hydrolysates have been studied. The best results are obtained for overliming + ethyl acetate extraction + activated charcoal adsorption for phenolics for eucalyptus wood hydrolysates [147] and the use of activated charcoal or diatomaceous earths + anionic resin in the case of furans for eucalyptus wood [50]. In addition, using flocculation + resin-wafer electrodeionisation (RW-EDI), good results in all of the inhibitors have been obtained, with a removal of 60%–74% of furans, 77% acetic acid, and 97% of sulphuric acid when Ponderosa pine wood hydrolysate is used as the raw material [43]. Both processes, flocculation + resin-wafer electrodeionisation, are explained in the following section.3.8. Other Processes
3.8.1. Steam Stripping
3.8.2. Reducing Agents
3.8.3. Other Membrane Processes
3.8.4. Aqueous Two-Phase Extraction
3.8.5. Supercritical Extraction
3.8.6. Advanced Oxidation Processes
3.8.7. Polyelectrolytic Flocculation
4. Application to Lignocellulosic Materials Derived from SO2-Based Processes
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Wierckx, N.; Koopman, F.; Ruijssenaars, H.J.; de Winde, J.H. Microbial degradation of furanic compounds: Biochemistry, genetics, and impact. Appl. Microbiol. Biotechnol. 2011, 92, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C. Treatment of rice straw hemicellulosic hydrolysates with advanced oxidative processes: A new and promising detoxification method to improve the bioconversion process. Biotechnol. Biofuels 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. Biotechnology 2015, 5, 337–353. [Google Scholar] [CrossRef]
- Rowell, R.M. The use of biomass to produce bio-based composites and building materials. In Advances in Biorefineries. Biomass and Waste Supply Chain Exploitation; Waldron, K., Ed.; Elsevier: Hong Kong, China, 2014; pp. 803–818. [Google Scholar]
- Fava, F.; Totaro, G.; Diels, L.; Reis, M.; Duarte, J.; Carioca, O.B.; Poggi-Varaldo, H.M.; Sommer Ferreira, B. Biowaste biorefinery in Europe: Opportunities and research & development needs. New Biotechnol. 2015, 32, 100–108. [Google Scholar]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Slininger, P.J.; Gorsich, S.W. Enhanced biotransformation of furfural and hydroxymethylfurfural by newly developed ethanologenic yeast strains. Appl. Biochem. Biotechnol. 2005, 121–124, 451–460. [Google Scholar] [CrossRef]
- Okuda, N.; Soneura, M.; Ninomiya, K.; Katakura, Y.; Shioya, S. Biological detoxification of waste house wood hydrolysate using Urebacillus thermosphaericus for bioethanol production. J. Biosci. Bioeng. 2008, 106, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Venditti, R.A.; Jameel, H.; Kenealy, W.R. Detoxification of woody hydrolyzates with activated carbon for bioconversion to ethanol by the thermophilic anaerobic bacterium Thermoanaerobacterium saccharolyticum. Biomass Bioenergy 2011, 35, 626–636. [Google Scholar] [CrossRef]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Sixta, H. Handbook of Pulp; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Huang, H.-J.; Ramaswamy, S.H.; Tschirner, U.W.; Ramarao, B.V. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Boussarsar, H.; Rogé, B.; Mathlouthi, M. Optimization of sugarcane bagasse conversion by hydrothermal treatment for the recovery of xylose. Bioresour. Technol. 2009, 100, 6537–6542. [Google Scholar] [CrossRef] [PubMed]
- Llano, T.; Rueda, C.; Quijorna, N.; Blanco, A.; Coz, A. Study of the delignification of hardwood chips in a pulping process for sugar production. J. Biotechnol. 2012, 162, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Lim, W.-S.; Lee, J.-W. Improvement of ethanol fermentation from lignocellulosic hydrolysates by the removal of inhibitors. J. Ind. Eng. Chem. 2013, 19, 2010–2015. [Google Scholar] [CrossRef]
- Zhou, F.; Wanga, C.; Wei, J. Simultaneous acetic acid separation and monosaccharide concentration by reverse osmosis. Bioresour. Technol. 2013, 131, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Bref Document—IPPC. Reference Document on Best Available Techniques in the Pulp and Paper Industry; European Commission: Seville, Spain, 2015. [Google Scholar]
- Toledano, A.; Serrano, L.; Garcia, A.; Mondragon, I.; Labidi, J. Comparative study of lignin fractionation by ultrafiltration and selective precipitation. Chem. Eng. J. 2010, 157, 93–99. [Google Scholar] [CrossRef]
- Chandel, A.K.; Singh, O.V.; Rao, L.V. Biotechnological applications of hemicellulosic derived sugars: State-of-the-art. In Sustainable Biotechnology: Renewable Resources and New Perspectives; Singh, O.V., Harvey, S.P., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 63–81. [Google Scholar]
- Chandel, A.K.; da Silva, S.S.; Singh, O.V. Detoxification of lignocellulose hydrolysates: Biochemical and metabolic engineering toward white biotechnology. Bioenergy Res. 2013, 6, 388–401. [Google Scholar] [CrossRef]
- Holladay, J.E.; Bozell, J.J.; White, J.F.; Johnson, D. Top Value Added Chemicals from Biomass: Volume II—Results of Screening for Potential Candidates from Biorefinery Lignin; US Department of Energy: New York, NY, USA, 2007. [Google Scholar]
- Zhang, X.; Tu, M.; Paice, M.G. Routes to potential bioproducts from lignocellulosic biomass lignin and hemicelluloses. Bioenergy Res. 2011, 4, 246–257. [Google Scholar] [CrossRef]
- Bizzari, S.N.; Janshekar, H.; Yokose, K. Lignosulfonates; CEH Marteking Research Report; SRI Consulting: Englewood, CO, USA, 2010. [Google Scholar]
- IEA. IEA Bioenergy Annual Report 2009. Available online: http://www.ieabioenergy.com/wp-content/uploads/2013/10/IEA-Bioenergy-2009-Annual-Report.pdf (accessed on 7 March 2016).
- Moshkelani, M.; Marinova, M.; Perrier, M.; Paris, J. The forest biorefinery and its implementation in the pulp and paper industry: Energy overview. Appl. Therm. Eng. 2013, 50, 1427–1436. [Google Scholar] [CrossRef]
- Agbor, V.; Carere, C.; Cicek, N.; Sparling, R.; Levin, D. Biomass pretreatment for consolidated bioprocessing (CBP). In Advances in Biorefineries. Biomass and Waste Supply Chain Exploitation; Waldron, K., Ed.; Elsevier: Hong Kong, China, 2014; pp. 234–258. [Google Scholar]
- Harmsen, P.; Huijgen, W.; Bermudez, L.; Bakker, R. Literature review of physical and chemical pretreatment processes for lignocellulosic biomass. In Biosinergy; Wageningen UR Food & Biobased Research; Institute within the Legal Entity Stichting Dienst Landbouwkundig Onderzoek: Wageningen, The Netherlands, 2010. [Google Scholar]
- Lenihan, P.; Orozco, A.; O’Neill, E.; Ahmad, M.N.M.; Rooney, D.W.; Walker, G.M. Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J. 2010, 156, 395–403. [Google Scholar] [CrossRef]
- Xavier, A.M.R.B.; Correia, M.F.; Pereira, S.R.; Evtuguin, D.V. Second-generation bioethanol from eucalypt sulphite spent liquor. Bioresour. Technol. 2010, 101, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, M.; Ai, N.; Yu, F.; Ji, J. Kinetic model analysis of dilute sulfuric acid-catalyzed hemicellulose hydrolysis in sweet sorghum bagasse for xylose production. Ind. Crops Prod. 2012, 38, 81–86. [Google Scholar] [CrossRef]
- Martín, M.; Grossmann, I.E. Review: On the systematic synthesis of sustainable biorefineries. Ind. Eng. Chem. Res. 2013, 52, 3044–3064. [Google Scholar] [CrossRef]
- Liu, Z.L. Genomic adaptation of ethanologenic yeast to biomass conversion inhibitors. Appl. Microbiol. Biotechnol. 2006, 73, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates II: Inhibitors and mechanism of inhibition review. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Silva, S.S.; Singh, O.V. Detoxification of lignocellulosic hydrolysates for improved bioconversion of bioethanol. In Biofuel Production—Recent Developments and Prospects; Bernardes, M.A.S., Ed.; InTech: Rijeka, Hrvatska, 2011; pp. 225–246. [Google Scholar]
- Holm-Nielsen, J.B.; Ehimen, E.A. Biorefinery plant design, engineering and process optimization. In Advances in Biorefineries. Biomass and Waste Supply Chain Exploitation; Waldron, K., Ed.; Elsevier: Hong Kong, China, 2014; pp. 89–111. [Google Scholar]
- Pampulha, M.E.; Loureiro-Dias, M.C. Activity of glucolytic enzymes of Saccharomyces cerevisiae in the presence of acetic acid. Appl. Microbiol. Biotechnol. 1990, 34, 375–380. [Google Scholar] [CrossRef]
- Zaldivar, J.; Ingram, L.O. Effect of organic acids on the growth and fermentation of ethanologenic Escherichia coli LY01. Biotechnol. Bioeng. 1999, 66, 203–210. [Google Scholar] [CrossRef]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Inhibition of ethanol producing yeast and bacteria by degradation products produced during pretreatment of biomass. Appl. Microb. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Gurram, R.N.; Datta, S.; Lin, Y.J.; Snyder, S.W.; Menkhaus, T.J. Removal of enzymatic and fermentation inhibitory compounds from biomass slurries for enhanced biorefinery process efficiencies. Bioresour. Technol. 2011, 102, 7850–7859. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Grage, H.; Meinander, N.Q.; Hahn-Hägerdal, B. Main and interaction effects of acetic acid, furfural, and p-hydroxybenzoic acid on growth and ethanol productivity of yeasts. Biotechnol. Bioeng. 1999, 63, 46–55. [Google Scholar] [CrossRef]
- Zaldivar, J.; Martínez, A.; Ingram, L.O. Effect of selected aldehydes on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol. Bioeng. 1999, 65, 24–33. [Google Scholar] [CrossRef]
- Zaldivar, J.; Marínez, A.; Ingram, L.O. Effect of alcohol compounds found in hemicellulose hydrolysate on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol. Bioeng. 2000, 68, 524–530. [Google Scholar] [CrossRef]
- Helle, S.; Cameron, D.; Lam, J.; White, B.; Duff, S. Effect of inhibitory compounds found in biomass hydrolysates on growth and xylose fermentation by a genetically engineered strain of S. cerevisiae. Enzyme Microb. Technol. 2003, 33, 786–792. [Google Scholar] [CrossRef]
- Liu, Z.L.; Slininger, P.J.; Dien, B.S.; Berhow, M.A.; Kurtzman, C.P.; Gorsich, S.W. Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5-bis-hydroxymethylfuran. J. Ind. Microbiol. Biotechnol. 2004, 31, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Pienkos, P.T.; Zhang, E.M. Role of pretreatment and conditioning processes on toxicity of lignocellulosic biomass hydrolysates. Cellulose 2009, 16, 743–762. [Google Scholar] [CrossRef]
- Carvalho, G.B.M.; Mussatto, S.I.; Cândido, E.J.; Almeida e Silva, J.B. Comparison of different procedures for the detoxification of eucalyptus hemicellulosic hydrolysate for use in fermentative processes. J. Chem. Technol. Biotechnol. 2006, 81, 152–157. [Google Scholar] [CrossRef]
- Wang, B.; Feng, H. Detoxification of lignocellulosic hydrolysates. In Biofuels from Agricultural Wastes and Byproducts; Blaschek, H.P., Ezeji, T., Scheffran, J., Eds.; Wiley-Blackwellv: Ames, IA, USA, 2010; pp. 233–250. [Google Scholar]
- Qi, B.; Luo, J.; Chen, X.; Hang, X.; Wan, Y. Separation of furfural from monosaccharides by nanofiltration. Bioresour. Technol. 2011, 102, 7111–7118. [Google Scholar] [CrossRef] [PubMed]
- Canilha, L.; Chandel, A.K.; dos Santos Milessi, T.S.; Fernandes Antunes, F.A.; da Costa Freitas, W.L.; Almeida Felipe, M.G.; da Silva, S.S. Review article: Bioconversion of sugarcane biomass into ethanol: An overview about composition, pretreatmentmethods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Reimann, A.; Nilvebrant, N.O.; Jonsson, L.J. Comparison of different methods for the detoxification of lignocellulose hydrolyzates of spruce. Appl. Biochem. Biotechnol. 1999, 77–79, 91–103. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Santos, J.C.; Roberto, I.C. Effect of pH and activated charcoal adsorption on hemicellulosic hydrolysate detoxification for xylitol production. J. Chem. Technol. Biotechnol. 2004, 79, 590–596. [Google Scholar] [CrossRef]
- Horváth, I.; Sjöde, A.; Alriksson, B.; Jönsson, L.; Nilvebrant, N.-O. Critical conditions for improved fermentability during overliming of acid hydrolysates from spruce. Appl. Biochem. Biotechnol. 2005, 121–124, 1031–1044. [Google Scholar] [CrossRef]
- Chandel, A.K.; Kapoor, R.K.; Singh, A.; Kuhad, R.C. Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresour. Technol. 2007, 98, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Telli-Okur, M.; Eken-Saraçoglu, N. Fermentation of sunflower seed hull hydrolysate to ethanol by Pichia stipitis. Bioresour. Technol. 2008, 99, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Blaschek, H.P. Biomass conversion inhibitors and in situ detoxification. In Biomass to Biofuels: Strategies for Global Industries; Vertès, A.A., Qureshi, N., Blaschek, H.P., Yukawa, H., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2010; pp. 233–259. [Google Scholar]
- Carter, B.; Squillace, P.H.; Gilcrease, P.C.; Menkhaus, T.J. Detoxification of a lignocellulosic biomass slurry by soluble polyelectrolyte adsorption for improved fermentation efficiency. Biotechnol. Bioeng. 2011, 18, 2053–2060. [Google Scholar] [CrossRef] [PubMed]
- Tengborg, C.; Galbe, M.; Zacchi, G. Reduced inhibition of enzymatic hydrolysis of steam-pretreated softwood. Enzyme Microb. Technol. 2001, 28, 835–844. [Google Scholar] [CrossRef]
- Villarreal, M.L.M.; Prata, A.M.R.; Felipe, M.G.A.; Almeida, E.; Silva, J.B. Detoxification procedures of eucalyptus hemicellulose hydrolysate for xylitol production by Candida guilliermondii. Enzyme Microb. Technol. 2006, 40, 17–24. [Google Scholar] [CrossRef]
- Zhu, J.; Yong, Q.; Xu, Y.; Yu, S.H. Detoxification of corn stover prehydrolyzate by trialkylamine extraction to improve the ethanol production with Pichia stipitis CBS 5776. Bioresour. Technol. 2011, 102, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Alvarez, R.; Lettinga, G. The methanogenic toxicity of wastewater lignins and lignin related compounds. J. Chem. Technol. Biotechnol. 1991, 50, 443–455. [Google Scholar] [CrossRef]
- Larsson, S.; Palmqvist, E.; Hahn-Hägerdal, B.; Tengborg, C.; Stenberg, K.; Zacchi, G.; Nilvebrant, N. The generation of inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb. Technol. 1999, 24, 151–159. [Google Scholar] [CrossRef]
- Lewkowski, J. Synthesis, chemistry and applications of 5-hydroxymethylfurfural and its derivatives. ARKIVOC 2001, 1, 17–54. [Google Scholar]
- Liu, Z.L.; Moon, J.; Andersh, B.J.; Slininger, P.J.; Weber, S. Multiple gene mediated aldehyde reduction is a mechanism of in situ detoxification of furfural and HMF by ethanologenic yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2008, 81, 743–753. [Google Scholar] [PubMed]
- Heer, D.; Sauer, U. Identification of furfural as a key toxin in lignocellulosic hydrolysates and evolution of a tolerant yeast strain. Microb. Biotechnol. 2008, 1, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.M.; Bertilsson, M.; Gorwa-Grauslund, M.F.; Gorsich, S.; Liden, G. Metabolic effects of furaldehydes and impacts on biotechnological processes. Appl. Microbiol. Biotechnol. 2009, 82, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.H.; Thygesen, A.; Thomsen, A.B. Identification and characterization of fermentation inhibitors formed during hydrothermal treatment and following SSF of wheat straw. Appl. Microbiol. Biotechnol. 2009, 83, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Olsson, L.; Hahn-Hägerbal, B. Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb. Technol. 1996, 18, 312–331. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Gustafsson, L.; Niklasson, C.; Lindén, G. Physiological effects of 5-hydroxymethylfurfural on Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2000, 53, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, T.; Qureshi, N.; Blaschek, H. Butanol production from agricultural residues: Impact of degradation products on Clostridium beijerinckii growth and butanol fermentation. Biotechnol. Bioeng. 2007, 97, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, N.; Bhatnagar, R.; Viswanathan, L. Inhibition of glycolysis by furfural in Saccharomyces cerevisiae. Eur. J. Appl. Microbiol. Biotechnol. 1981, 11, 226–228. [Google Scholar] [CrossRef]
- Jing, X.; Zhang, X.; Bao, J. Inhibition performance of lignocellulose degradation products on industrial cellulase enzymes during cellulose hydrolysis. Appl. Biochem. Biotechnol. 2009, 159, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.J.; Gustafsson, L.; Niklasson, C.; Lidén, G. Conversion of furfural in aerobic and anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. J. Biosci. Bioeng. 1999, 87, 169–174. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Hong, Y.-K.; Jeong, G.-T. Detoxification of acidic catalyzed hydrolysate of Kappaphycus alvarezii (cottonii). Bioprocess Biosyst. Eng. 2012, 35, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.S.; Qian, K.; Usami, Y.; Lin, L.; Itokawa, H.; Hsu, C.; Morris-Natschke, S.L.; Lee, K.H. 5-Hydroxymethyl-2-furfural, a clinical trials agent for sickle cell anemia, and its mono/di-glucosides from classically processed steamed rehmanniae radix. J. Nat. Med. 2008, 62, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, M.Y.; Yao, Y.X.; Li, G.Y.; Cai, B.C. Protective effect of 5-hydroxymethylfurfural derived from processed Fructus Corni on human hepatocyte LO2 injured by hydrogen peroxide and its mechanism. J. Ethnopharmacol. 2010, 128, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Michail, K.; Matzi, V.; Maier, A.; Herwig, R.; Greilberger, J.; Juan, H.; Kunert, O.; Wintersteiger, R. Hydroxymethylfurfural: An enemy or a friendly xenobiotic? A bioanalytical approach. Anal. Bioanal. Chem. 2007, 387, 2801–2814. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.M.; Modig, T.; Petersson, A.; Hahn-Hägerdal, B.; Liden, G.; Gorwa-Grauslund, M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Niklasson, C.; Lidén, G. Acetic-acid—Friend or foe in anaerobic batch conversion of glucose to ethanol by Saccharomyces cerevisiae? Chem. Eng. Sci. 1997, 52, 2653–2659. [Google Scholar] [CrossRef]
- Heipieper, J.J.; Weber, F.J.; Sikkema, J.; Keweloh, H.; de Bont, J.A.M. Mechanism of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994, 12, 409–415. [Google Scholar] [CrossRef]
- Odeh, R.M.; Cornish, L.A. Natural antioxidants for the prevention of atherosclerosis. Pharmacotherapy 1995, 15, 648–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- German, J.B.; Walzem, R.L. The health benefits of wine. Annu. Rev. Nutr. 2000, 20, 561–593. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Seifried, H.E.; Anderson, D.E.; Fisher, E.I.; Milner, J.A. A review of the interaction among dietary antioxidants and reactive oxygen species. J. Nutr. Biochem. 2007, 18, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Amici, M.; Cuccioloni, M.; Angeletti, M.; Keller, J.N.; Eleuteri, A.M. Natural polyphenols as proteasome modulators and their role as anti-cancer compounds. FEBS J. 2008, 275, 5512–5526. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.J.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Halliwell, B. The wanderings of a free radical. Free Radic. Biol. Med. 2009, 46, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.L.; Moure, A.; Dominguez, H.; Parajó, J.C. Recovery, concentration and purification of phenolic compounds by adsorption: A review. J. Food Eng. 2011, 105, 1–27. [Google Scholar] [CrossRef]
- Martin, C.; Jönsson, L. Comparison of the resistance of industrial and laboratory strains of Saccharomyces and Zygosaccharomyces to lignocellulose-derived fermentation inhibitors. Enzyme Microb. Technol. 2003, 32, 386–395. [Google Scholar] [CrossRef]
- Nigam, J.N. Ethanol production from wheat straw hemicelluloses hydrolysate by Scheffersomyces stipitis. J. Biotechnol. 2001, 87, 17–27. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Martinez, A.; Rodriguez, M.E.; Wells, M.L.; York, S.W.; Preston, J.F.; Ingram, L.O. Detoxification of dilute acid hydrolysates of lignocellulose with lime. Biotechnol. Prog. 2001, 17, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Patiño, J.C.; Romero-García, J.M.; Ruiz, E.; Oliva, J.M.; Álvarez, C.; Romero, I.; Negro, M.J.; Castro, E. High solids loading pretreatment of olive tree pruning with dilute phosphoric acid for bioethanol production by Escherichia coli. Energy Fuels 2015, 29, 1735–1742. [Google Scholar] [CrossRef]
- Nilvebrant, N.-O.; Reimann, A.; Larsson, S.; Jönsson, L.J. Detoxification of lignocellulose hydrolysates with ion-exchange resins. Appl. Biochem. Biotechnol. 2001, 91–93, 35–49. [Google Scholar] [CrossRef]
- Alves, L.A.; Vitolo, M.; Felipe, M.G.A.; Almeida e Silva, J.B. Xylose reductase and xylitol dehydrogenase activities of Candida guilliermondii as a function of different treatments of sugarcane bagasse hemicellulosic hydrolysate employing experimental design. Appl. Biochem. Biotechnol. 2002, 98–100, 403–413. [Google Scholar] [CrossRef]
- Weil, J.R.; Dien, B.; Bothast, R.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R. Removal of fermentation inhibitors formed during pretreatment of biomass by polymeric adsorbents. Ind. Eng. Chem. Res. 2002, 41, 6132–6138. [Google Scholar] [CrossRef]
- Carvalho, W.; Canilha, L.; Mussatto, S.I.; Dragone, G.; Morales, M.L.V.; Solenzal, A.I.N. Detoxification of sugarcane bagasse hemicellulosic hydrolysate with ion-exchange resins for xylitol production by calcium alginate-entrapped cells. J. Chem. Technol. Biotechnol. 2004, 79, 863–868. [Google Scholar] [CrossRef]
- Carvalho, R.J.; Marton, J.M.; Silva, F.; Felipe, M.G. Avaliação do sistema combinado de tratamento do hidrolisado hemicelulósico de bagaço de cana-de-açúcar com carvão ativo e resinas de troca iônica para sua utilização como meio de fermentação. Rev. Anal. 2005, 18, 48–55. [Google Scholar]
- Xie, Y.; Phelps, D.; Lee, C.-H.; Sedlak, M.; Ho, N.; Wang, N.-H.L. Comparison of two adsorbents for sugar recovery from biomass hydrolyzate. Ind. Eng. Chem. Res. 2005, 44, 6816–6823. [Google Scholar] [CrossRef]
- Ranjan, R.S.; Thust, S.; Gounaris, C.E.; Woo, M.; Floudas, C.A.; von Keitz, M.; Valentas, K.J.; Wei, J.; Tsapatsis, M. Adsorption of fermentation inhibitors from lignocellulosic biomass hydrolyzates for improved ethanol yield and valueadded product recovery. Microporous Mesoporous Mater. 2009, 122, 143–148. [Google Scholar] [CrossRef]
- Zhuang, J.; Liu, Y.; Wu, Z.; Sun, Y.; Lin, L. Hydrolysis of wheat straw hemicellulose and detoxification of the hydrolysate for xylitol production. BioResources 2009, 4, 674–686. [Google Scholar]
- Lopez-Linares, J.C.; Cara-Corpas, C.; Ruiz-Ramos, E.; Moya-Vilar, M.; Castro-Galiano, E.; Romero-Pulido, I. Hemicellulose-derived sugars solubilisation of rape straw. Cofermentation of pentoses and hexoses by Escherichia coli. Span. J. Agric. Res. 2015, 13. [Google Scholar] [CrossRef]
- Christopher, M.; Anusree, M.; Mathew, A.K.; Nampoothiri, K.M.; Sukumaran, R.K.; Pandey, A. Detoxification of acidic biorefinery waste liquor for production of high value amino acid. Bioresour. Technol. 2016, 213, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Llano, T.; Alexandri, M.; Koutinas, A.; Gardeli, C.H.R.; Papapostolou, H.; Coz, A.; Quijorna, N.; Andres, A.; Komaitis, M. Liquid-liquid extraction of phenolic compounds from spent sulphite liquor. Waste Biomass Valor 2015, 6, 1149–1159. [Google Scholar] [CrossRef]
- Converti, A.; Domínguez, J.M.; Perego, P.; Silva, S.S.; Zilli, M. Wood hydrolysis and hydrolysate detoxification for subsequent xylitol production. Chem. Eng. Technol. 2000, 23, 1013–1020. [Google Scholar] [CrossRef]
- Rodrigues, R.C.L.B.; Felipe, M.G.A.; Almeida e Silva, J.B.; Vitolo, M.; Gómez, P.V. The influence of pH, temperature and hydrolyzate concentration on the removal of volatile and nonvolatile compounds from sugarcane bagasse hemicellulosic hydrolyzate treated with activated charcoal before or after vacuum evaporation. Braz. J. Chem. Eng. 2001, 18, 299–311. [Google Scholar] [CrossRef]
- López, M.J.; Nichols, N.N.; Dien, B.S.; Moreno, J.; Bothast, R.J. Isolation of microorganisms for biological detoxification of lignocellulosic hydrolysates. Appl. Microbiol. Biotechnol. 2004, 64, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Nichols, N.N.; Dien, B.S.; Cotta, M.A. Fermentation of bioenergy crops into ethanol using biological abatement for removal of inhibitors. Bioresour. Technol. 2010, 101, 7545–7550. [Google Scholar] [CrossRef] [PubMed]
- Alriksson, B.; Cavka, A.; Jönsson, L.J. Improving the fermentability of enzymatic hydrolysates of lignocelluloses through chemical in-situ detoxification with reducing agents. Bioresour. Technol. 2011, 102, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Anish, R.; Rao, M. Bioethanol from lignocellulosic biomass part III hydrolysis and fermentation. In Handbook of Plant-Based Biofuels; Pandey, A., Ed.; CRC Press: Portland, OR, USA, 2009; pp. 159–173. [Google Scholar]
- Sainio, T.; Turku, I.; Heinonen, J. Adsorptive removal of fermentation inhibitors from concentrated acid hydrolyzates of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 6048–6057. [Google Scholar] [CrossRef] [PubMed]
- Schirmer-Michel, Â.C.; Flôres, S.H.; Hertz, P.F.; Matos, G.S.; Ayub, M.A.Z. Production of ethanol from soybean hull hydrolysate by osmotolerant Candida guilliermondii NRRL Y-2075. Bioresour. Technol. 2008, 99, 2898–2904. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Zong, M.-H.; Wu, H.; Liu, Q.-P. Microbial oil production from rice straw hydrolysate by Trichosporon fermentans. Bioresour. Technol. 2009, 100, 4535–4538. [Google Scholar] [CrossRef] [PubMed]
- Mateo, S.; Roberto, I.C.; Sánchez, S.; Moya, A.J. Detoxification of hemicellulosic hydrolyzate from olive tree pruning residue. Ind. Crops Prod. 2013, 49, 196–203. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Lopes, S.; Parajó, J.C.; Pereira, H.; Gírio, F.M. Evaluation of the detoxification of brewery’s spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Process Biochem. 2005, 40, 1215–1223. [Google Scholar] [CrossRef]
- Millati, R.; Niklasson, C.; Taherzadeh, M.J. Effect of pH, time and temperature of overliming on detoxification of dilute-acid hydrolyzates for fermentation by Saccharomyces cerevisiae. Proc. Biochem. 2002, 38, 515–522. [Google Scholar] [CrossRef]
- Carlos Martín, C.; Galbe, M.; Wahlbom, C.F.; Hahn-Hägerdal, B.; Jönsson, L.J. Ethanol production from enzymatic hydrolysates of sugarcane bagasse using recombinant xylose-utilising Saccharomyces cerevisiae. Enzyme Microb. Technol. 2002, 31, 274–282. [Google Scholar] [CrossRef]
- Alriksson, B.; Sjöde, A.; Nilvebrant, N.O.; Jönsson, L.J. Optimal conditions for alkaline detoxification of dilute-acid lignocellulose hydrolysates. Appl. Biochem. Biotechnol. 2006, 130, 599–611. [Google Scholar] [CrossRef]
- Andary, J.; Maalouly, J.; Ouaini, R.; Chebib, H.; Rutledge, D.N.; Ouaini, N. Application of 2D correlation spectroscopy on olive stones acid hydrolysates: Effect of overliming. Chemom. Intell. Lab. 2012, 113, 58–67. [Google Scholar] [CrossRef]
- Purwadi, R.; Niklasson, C.; Taherzadeh, M.J. Kinetic study of detoxification of dilute-acid hydrolyzates by Ca(OH)2. J. Biotechnol. 2004, 114, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Berson, R.E.; Young, J.S.; Hanley, T.R. Reintroduced solids increase inhibitor levels in a pretreated corn stover hydrolysate. Appl. Biochem. Biotechnol. 2006, 129–132, 612–620. [Google Scholar] [CrossRef]
- Datta, S.; Lin, Y.J.; Snyder, S.W. Current and emerging separations technologies in biorefining. In Advances in Biorefineries. Biomass and Waste Supply Chain Exploitation; Waldron, K., Ed.; Elsevier: Hong Kong, China, 2014; pp. 112–151. [Google Scholar]
- Eken-Saraçoglu, N.; Arslan, Y. Comparison of different pretreatments in ethanol fermentation using corn cob hemicellulosic hydrolysate with Pichia stipitis and Candida shehatae. Biotechnol. Lett. 2000, 22, 855–858. [Google Scholar] [CrossRef]
- Ribeiro, M.H.L.; Lourenço, P.A.S.; Monteiro, J.P.; Ferreira-Dias, S. Kinetics of selective adsorption of impurities from a crude vegetable oil in hexane to activated earths and carbons. Eur. Food Res. Technol. 2001, 213, 132–138. [Google Scholar]
- Miyafuji, H.; Danner, H.; Neureiter, M.; Thomasser, C.; Bvochora, J.; Szolar, O.; Rudolf, B. Detoxification of wood hydrolysates with wood charcoal for increasing the fermentability of hydrolysates. Enzyme Microb. Technol. 2003, 32, 396–400. [Google Scholar] [CrossRef]
- De Mancilha, I.M.; Karim, M.N. Evaluation of ion exchange resins for removal of inhibitory compounds from corn stover hydrolyzate for xylitol fermentation. Biotechnol. Prog. 2003, 19, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Griffin, G.J.; Shu, L. Solvent extraction and purification of sugars from hemicellulose hydrolysates using boronic acid carriers. J. Chem. Technol. Biotechnol. 2004, 79, 505–511. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, Y.; Zhang, L.; Yong, Q.; Xu, Y.; Li, X.; Lian, Z.; Yu, S. Sodium hydroxide regeneration of trialkylamine extractant containing inhibitors from corn stover prehydrolyzate by liquid-liquid extraction. Sep. Purif. Technol. 2014, 126, 39–43. [Google Scholar] [CrossRef]
- Wilson, J.J.; Deschatelets, L.; Nishikawa, N. Comparative fermentability of enzymatic and acid hydrolysates of steam-pretreated aspenwood hemicellulose by Pichia stipitis CBS 5776. Appl. Microbiol. Biotechnol. 1989, 31, 592–596. [Google Scholar] [CrossRef]
- Frazer, F.R.; McCaskey, T.A. Wood hydrolyzate treatments for improved fermentation of wood sugars to 2,3-butanediol. Biomass 1989, 18, 31–42. [Google Scholar] [CrossRef]
- Dhamole, P.B.; Wang, B.; Feng, H. Detoxification of corn stover hydrolysate using surfactant-based aqueous two phase system. J. Chem. Technol. Biotechnol. 2013, 88, 1744–1749. [Google Scholar] [CrossRef]
- Samaddar, P.; Sen, K. Cloud point extraction: A sustainable method of elemental preconcentration and speciation. J. Ind. Eng. Chem. 2014, 4, 1209–1219. [Google Scholar] [CrossRef]
- Pinelo, M.; Jonsson, G.; Meyer, A.S. Membrane technology for purification of enzymatically produced oligosaccharides: Molecular and operational features affecting performance. Sep. Purif. Technol. 2009, 70, 1–11. [Google Scholar] [CrossRef]
- Li, N.N.; Fane, A.G.; Ho, W.S.; Matsuura, T. Advanced Membrane Technology and Applications; Wiley-AIChE: Hoboken, NJ, USA, 2008. [Google Scholar]
- Koivula, E.; Kallioinen, M.; Preis, S.; Testova, L.; Sixta, H.; Mänttäri, M. Evaluation of various pretreatment methods to manage fouling in ultrafiltration of wood hydrolysates. Sep. Purif. Technol. 2011, 83, 50–56. [Google Scholar] [CrossRef]
- Weng, Y.-H.; Wei, H.-J.; Tsai, T.-Y.; Lin, T.-H.; Wei, T.-Y.; Guo, G.-L.; Huang, C.H.-P. Separation of furans and carboxylic acids from sugars in dilute acid rice straw hydrolyzates by nanofiltration. Bioresour. Technol. 2010, 101, 4889–4894. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Wang, Y.; Ji, X.; Zhang, L.; Mi, X.; Huang, H. Removal of inhibitors from lignocellulosic hydrolyzates by vacuum membrane distillation. Bioresour. Technol. 2013, 144, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Brás, T.; Guerra, V.; Torrado, I.; Lourenço, P.; Carvalheiro, F.; Duarte, L.C.; Neves, L.A. Detoxification of hemicellulosic hydrolysates from extracted olive pomace by diananofiltration. Process Biochem. 2014, 49, 173–180. [Google Scholar] [CrossRef]
- Nguyen, N.; Fargues, C.; Lewandowsky, R.; Guiga, W.; Lameliose, M.L. Assessing nanofiltration and reverse osmosis for the detoxification of fermentable solutions. Proc. Eng. 2012, 44, 1476–1478. [Google Scholar] [CrossRef]
- Kim, J.H.; Na, J.-G.; Yang, J.-W.; Chang, Y.K. Separation of galactose, 5-hydroxymethylfurfural and levulinic acid in acid hydrolysate of agarose by nanofiltration and electrodialysis. Bioresour. Technol. 2013, 140, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Wallberg, O.; Linde, M.; Jönsson, A.-S. Extraction of lignin and hemicelluloses from kraft black liquor. Desalination 2006, 199, 413–414. [Google Scholar] [CrossRef]
- Sklavounos, E.; Iakovlev, M.; van Heiningen, A. Study on conditioning of SO2-Ethanol-Water spent liquor from spruce chips/softwood biomass for ABE fermentation. Ind. Eng. Chem. Res. 2013, 52, 4351–4359. [Google Scholar] [CrossRef]
- Parajó, J.C.; Dominguez, H.; Dominguez, J.M. Xylitol production from Eucalyptus wood hydrolysates extracted with organic solvents. Process Biochem. 1997, 32, 599–604. [Google Scholar] [CrossRef]
- Nilsson, A.; Gorwa-Grauslund, M.F.; Hahn-Hägerdal, B.; Lidén, G. Cofactor dependence in furan reduction by Saccharomyces cerevisiae in fermentation of acid-hydrolyzed lingocellulose. Appl. Environ. Microbiol. 2005, 71, 7866–7871. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Trinh, L.T.P.; Lee, H.-J. Removal of inhibitors from a hydrolysate of lignocellulosic biomass using electrodialysis. Sep. Purif. Technol. 2014, 122, 242–247. [Google Scholar] [CrossRef]
- Sreenath, H.; Jeffries, T.W. Production of ethanol from wood hydrolyzate by yeasts. Bioresour. Technol. 2000, 72, 253–260. [Google Scholar] [CrossRef]
- Lin, Y.J.; Henry, M.P.; Snyder, S.W. Electronically and Ionically Conductive Porous Material and Method for Manufacture of Resin Wafers Therefrom. U.S. Patent 7,452,920, 17 September 2004. [Google Scholar]
- Arora, M.B.; Hestekin, J.A.; Snyder, S.W.; Martin, E.J.; Donnelly, M.I.; Sanville-Millard, C.; Lin, Y.J. The separative bioreactor: A continuous separation process for the simultaneous production and direct capture of organic acids. Sep. Sci. Technol. 2007, 42, 2519–2538. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Henry, M.P.; Snyder, S.W.; Martin, E.S.; Arora, M.B.; de la Garza, L. Devices Using Resin Wafers and Applications Thereof. U.S. Patent 7,507,318, 17 March 2005. [Google Scholar]
- Datta, S.; Henry, M.P.; Ahmad, S.F.; Snyder, S.W.; Lin, Y.J. Removal of salt impurities from glycerin using electrodeionization technique. In Proceedings of the AIChE National Meeting, Tampa, Finland, 27–30 April 2009.
- Datta, S.; Lin, Y.J.; Schell, D.J.; Millard, C.S.; Ahmad, S.F.; Henry, M.P.; Gillenwater, P.; Fracaro, A.T.; Moradia, A.; Gwarnicki, Z.P.; et al. Removal of acidic impurities from corn stover hydrolysate liquor by resin wafer based electrodeionization. Ind. Eng. Chem. Res. 2013, 52, 13777–13784. [Google Scholar] [CrossRef]
- Lin, Y.J.; Snyder, S.W.; Trachtenberg, M.C.; Cowan, R.M.; Datta, S. Carbon Dioxide Capture Using Resin-Wafer Electrodeionization. U.S. Patent 8,506,784, 29 May 2009. [Google Scholar]
- Hasmann, F.A.; Santos, V.C.; Gurpilhares, D.B.; Pessoa-Junior, A.; Roberto, I. Aqueous two-phase extraction using thermoseparating copolymer: A new system for phenolic compounds removal from hemicelullosic hydrolysates. J. Chem. Technol. Biotechnol. 2008, 83, 167–173. [Google Scholar] [CrossRef]
- Persson, P.; Larsson, S.; Jönsson, N.J.; Nilvebrant, N.-O.; Sivik, B.; Munteanu, F.; Thörneby, L.; Gorton, L. Supercritical fluid extraction of a lignocellulosic hydrolysate of spruce for detoxification and to facilitate analysis of inhibitors. Biotechnol. Bioeng. 2002, 79, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Mohamed, H.; Limem, E.; Bensalah, N. Degradation and mineralization of organic pollutants contained in actual pulp and paper mill wastewaters by a UV/H2O2 process. Ind. Eng. Chem. 2009, 48, 3370–3379. [Google Scholar] [CrossRef]
- Carter, B.; Gilcrease, P.C.; Menkhaus, T.J. Removal and recovery of furfural, 5-hydroxymethylfurfural, and acetic acid from aqueous solutions using a soluble polyelectrolyte. Biotechnol. Bioeng. 2011, 108, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.R.; Anderson, J.; Gilcrease, P.C.; Menkhuas, T.J. Enhanced solid-liquid clarification of lignocellulosic slurries using polyelectrolyte flocculating agents. Biomass Bioenergy 2011, 35, 391–401. [Google Scholar] [CrossRef]
- Yu, M.; Wang, G.; Liu, C.; Ruhan, A. Precipitation of lignosulphonates from SPORL liquid by calcium hydroxide treatment. BioResources 2012, 7, 868–877. [Google Scholar]
- Sixta, H.; Iakovlev, M.; Testova, L.; Roselli, A.; Hummel, M.; Borrega, M.; van Heiningen, A.; Froschauer, C.; Schottenberger, H. Novel concepts of dissolving pulp production. Cellulose 2013, 20, 1547–1561. [Google Scholar] [CrossRef]
- Maican, E.; Teixeira, J.A.; Ferdeş, M.; Coz, A. Energy efficient technologies for lignocellulosic ethanol production. INMATEH Agric. Eng. 2015, 2015, 435–446. [Google Scholar]
- Zhu, W.; Zhu, J.; Gleisner, R.; Pan, X. On energy consumption for size-reduction and yields from subsequent enzymatic saccharification of pretreated lodgepole pine. Bioresour. Technol. 2010, 101, 2782–2792. [Google Scholar] [CrossRef] [PubMed]
- Uihlein, A.; Schebec, L. Environmental impacts of a lignocellulose feedstock biorefinery system: An assessment. Biomass Bioenergy 2009, 33, 793–802. [Google Scholar] [CrossRef]
- Marques, A.P.; Evtuguin, D.V.; Magina, S.; Amado, F.M.L.; Prates, A. Chemical composition of spent liquors from acidic magnesium-based sulphite pulping of Eucalyptus globulus. J. Wood. Chem. Technol. 2009, 29, 322–336. [Google Scholar] [CrossRef]
- Hocking, M.B. Vanillin: Synthetic flavoring from spent sulfite liquor. J. Chem. Educ. 1997, 74, 1055–1059. [Google Scholar] [CrossRef]
- Plank, J. Applications of biopolymers and other biotechnological products in building materials. Appl. Microbiol. Biotechnol. 2004, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Smook, G.A. Handbook for Pulp & Paper Technologists, 3rd ed.; Angus Wilde Publications: Vancouver, BC, Canada, 2002. [Google Scholar]
- Pereira, S.R.; Portugal-Nunes, D.-N.; Evtuguin, D.V.; Serafim, L.S.; Xavier, A.M.R.B. Advances in ethanol production from hardwood spent sulphite liquors. Process Biochem. 2013, 48, 272–282. [Google Scholar] [CrossRef]
- Guo, X.; Cavka, A.; Jönsson, L.F.; Hong, F. Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microb. Cell Fact. 2013. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Ivanuša, Š.; Evtuguin, D.V.; Serafim, L.S.; Xavier, A.M.R.B. Biological treatment of eucalypt spent sulphite liquors: A way to boost the production of second generation bioethanol. Bioresour. Technol. 2012, 103, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Restolho, J.A.; Prates, A.; de Pinho, M.N.; Afonso, M.D. Sugars and lignosulphonates recovery from eucalyptus spent sulphite liquor by membrane processes. Biomass Bioenergy 2009, 33, 1558–1566. [Google Scholar] [CrossRef]
- Fernandes, D.L.A.; Silva, C.M.; Xavier, A.M.R.B.; Evtuguin, D.V. Fractionation of sulphite spent liquor for biochemical processing using ion exchange resins. J. Biotechnol. 2012, 162, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.K.; Todi, R.K.; Tiwar, M.; Bhattacharjee, C.; Bhattacharjee, S.; Datta, S. Studies on ultrafiltration of spent sulfite liquor using various membranes for the recovery of lignosulphonates. Desalination 2005, 174, 287–297. [Google Scholar] [CrossRef]
- Madsen, R.F.; Nielsen, W.K. Ultrafiltration of bleach effluents as an example of waste-water treatment by ultrafiltration. Desalination 1978, 24, 141–154. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; García, A.; Coz, A.; Labidi, J. Spent sulphite liquor fractionation into lignosulphonates and fermentable sugars by ultrafiltration. Sep. Purif. Technol. 2015, 152, 172–179. [Google Scholar] [CrossRef]
- Takahashi, S.; Tanifuji, K.; Shiell, K.; Fatehi, P.; Jahan, M.S.; Ohi, H.; Yonghao, N. Removal of acetic acid from spent sulfite liquor using anion exchange resin for effective xylose fermentation with Pichia stipitis. BioResources 2013, 8, 2417–2428. [Google Scholar] [CrossRef]
- Alexandri, M.; Papapostolou, H.; Vlysidis, A.; Gardeli, C.; Komaitis, M.; Papnikolaou, S.; Kouticas, A.A. Extration of phenolic compounds and succinic acid production from spent sulphite liquor. J. Chem. Technol. Biotechnol. 2016. [Google Scholar] [CrossRef]

 corn stover [63],
corn stover [63],  eucalyptus wood [50,62],
eucalyptus wood [50,62],  rice straw [37,117],
rice straw [37,117],  soybean hulls [116], and
soybean hulls [116], and  olive tree pruning residue [118] hydrolysates. The number included in the x axis is related to the reference number. Cell: Cellobiose, M: Mannose, L: Levulinic acid.
olive tree pruning residue [118] hydrolysates. The number included in the x axis is related to the reference number. Cell: Cellobiose, M: Mannose, L: Levulinic acid.
 corn stover [63],
corn stover [63],  eucalyptus wood [50,62],
eucalyptus wood [50,62],  rice straw [37,117],
rice straw [37,117],  soybean hulls [116], and
soybean hulls [116], and  olive tree pruning residue [118] hydrolysates. The number included in the x axis is related to the reference number. Cell: Cellobiose, M: Mannose, L: Levulinic acid.
olive tree pruning residue [118] hydrolysates. The number included in the x axis is related to the reference number. Cell: Cellobiose, M: Mannose, L: Levulinic acid.
 olive residues [97,118],
olive residues [97,118],  brewery’s spent grain [119],
brewery’s spent grain [119],  sugarcane bagasse [57,113],
sugarcane bagasse [57,113],  rice straw [117],
rice straw [117],  spruce [113,120],
spruce [113,120],  and Kappaphycus alvarezii [77] hydrolysates. The number included in the x axis is related to the reference number.
and Kappaphycus alvarezii [77] hydrolysates. The number included in the x axis is related to the reference number.
 olive residues [97,118],
olive residues [97,118],  brewery’s spent grain [119],
brewery’s spent grain [119],  sugarcane bagasse [57,113],
sugarcane bagasse [57,113],  rice straw [117],
rice straw [117],  spruce [113,120],
spruce [113,120],  and Kappaphycus alvarezii [77] hydrolysates. The number included in the x axis is related to the reference number.
and Kappaphycus alvarezii [77] hydrolysates. The number included in the x axis is related to the reference number.
 olive residues [97,118,123],
olive residues [97,118,123],  brewery’s spent grain [119],
brewery’s spent grain [119],  sugarcane bagasse [57,96,113,121],
sugarcane bagasse [57,96,113,121],  rice straw [117],
rice straw [117],  spruce [56,65,113,120,122], and
spruce [56,65,113,120,122], and  synthetic [124] hydrolysates. The number included in the x axis is related to the reference number.
synthetic [124] hydrolysates. The number included in the x axis is related to the reference number.
 olive residues [97,118,123],
olive residues [97,118,123],  brewery’s spent grain [119],
brewery’s spent grain [119],  sugarcane bagasse [57,96,113,121],
sugarcane bagasse [57,96,113,121],  rice straw [117],
rice straw [117],  spruce [56,65,113,120,122], and
spruce [56,65,113,120,122], and  synthetic [124] hydrolysates. The number included in the x axis is related to the reference number.
synthetic [124] hydrolysates. The number included in the x axis is related to the reference number.
 olive residues [97,118,123],
olive residues [97,118,123],  brewery’s spent grain [119],
brewery’s spent grain [119],  Kappaphycus alvarezii [77],
Kappaphycus alvarezii [77],  sugarcane bagasse [113],
sugarcane bagasse [113],  rice straw [117], and
rice straw [117], and  spruce [120] hydrolysates. The number included in the x axis is related to the reference number.
spruce [120] hydrolysates. The number included in the x axis is related to the reference number.
 olive residues [97,118],
olive residues [97,118],  sugarcane bagasse [57,96,113,121], and
sugarcane bagasse [57,96,113,121], and  spruce [56,65,113,120,122] hydrolysates. The number included in the x axis is related to the reference number.
spruce [56,65,113,120,122] hydrolysates. The number included in the x axis is related to the reference number.
 Kappaphycus alvarezii [77],
Kappaphycus alvarezii [77],  olive residues [97,123],
olive residues [97,123],  rice straw [117],
rice straw [117],  spruce [120], and
spruce [120], and  synthetic [124] hydrolysates. The number included in the x axis is related to the reference number. A: Arabinose.
synthetic [124] hydrolysates. The number included in the x axis is related to the reference number. A: Arabinose.
 Kappaphycus alvarezii [77],
Kappaphycus alvarezii [77],  olive residues [97,123],
olive residues [97,123],  rice straw [117],
rice straw [117],  spruce [120], and
spruce [120], and  synthetic [124] hydrolysates. The number included in the x axis is related to the reference number. A: Arabinose.
synthetic [124] hydrolysates. The number included in the x axis is related to the reference number. A: Arabinose.
 olive residues [97,118],
olive residues [97,118],  Kappaphycus alvarezii [77],
Kappaphycus alvarezii [77],  brewery’s spent grain [119],
brewery’s spent grain [119],  sugarcane bagasse [96,113,121], and
sugarcane bagasse [96,113,121], and  spruce [56,65,113,120,122] hydrolysates. The number included in the x axis is related to the reference number.
spruce [56,65,113,120,122] hydrolysates. The number included in the x axis is related to the reference number.
 olive residues [97,118],
olive residues [97,118],  Kappaphycus alvarezii [77],
Kappaphycus alvarezii [77],  brewery’s spent grain [119],
brewery’s spent grain [119],  sugarcane bagasse [96,113,121], and
sugarcane bagasse [96,113,121], and  spruce [56,65,113,120,122] hydrolysates. The number included in the x axis is related to the reference number.
spruce [56,65,113,120,122] hydrolysates. The number included in the x axis is related to the reference number.
 hardwood [10,62],
hardwood [10,62],  brewery’s spent grain [119],
brewery’s spent grain [119],  soybean hulls [116],
soybean hulls [116],  sugarcane bagasse [57],
sugarcane bagasse [57],  Rape straw [106],
Rape straw [106],  Kappaphycus alvarezii [77], and
Kappaphycus alvarezii [77], and  olive residues [118] hydrolysates. The number included in the x axis is related to the reference number. M: Mannose, Gal: Galactose.
olive residues [118] hydrolysates. The number included in the x axis is related to the reference number. M: Mannose, Gal: Galactose.
 hardwood [10,62],
hardwood [10,62],  brewery’s spent grain [119],
brewery’s spent grain [119],  soybean hulls [116],
soybean hulls [116],  sugarcane bagasse [57],
sugarcane bagasse [57],  Rape straw [106],
Rape straw [106],  Kappaphycus alvarezii [77], and
Kappaphycus alvarezii [77], and  olive residues [118] hydrolysates. The number included in the x axis is related to the reference number. M: Mannose, Gal: Galactose.
olive residues [118] hydrolysates. The number included in the x axis is related to the reference number. M: Mannose, Gal: Galactose.
 Picea abies [98],
Picea abies [98],  sugarcane bagasse [57,101],
sugarcane bagasse [57,101],  corn stover [130],
corn stover [130],  brewery’s spent grain [119],
brewery’s spent grain [119],  rice straw [117],
rice straw [117],  Eucalyptus grandis [62],
Eucalyptus grandis [62],  and rape straw [106] hydrolysates. The number included in the x axis is related to the reference number.
and rape straw [106] hydrolysates. The number included in the x axis is related to the reference number.
 Picea abies [98],
Picea abies [98],  sugarcane bagasse [57,101],
sugarcane bagasse [57,101],  corn stover [130],
corn stover [130],  brewery’s spent grain [119],
brewery’s spent grain [119],  rice straw [117],
rice straw [117],  Eucalyptus grandis [62],
Eucalyptus grandis [62],  and rape straw [106] hydrolysates. The number included in the x axis is related to the reference number.
and rape straw [106] hydrolysates. The number included in the x axis is related to the reference number.
 Picea abies [98],
Picea abies [98],  sugarcane bagasse [57,101],
sugarcane bagasse [57,101],  corn stover [130],
corn stover [130],  brewery’s spent grain [119],
brewery’s spent grain [119],  rice straw [117],
rice straw [117],  Eucalyptus grandis [62], and
Eucalyptus grandis [62], and  rape straw [106] hydrolysates. The number included in the x axis is related to the reference number. F: Furans.
rape straw [106] hydrolysates. The number included in the x axis is related to the reference number. F: Furans.
 Picea abies [98],
Picea abies [98],  sugarcane bagasse [57,101],
sugarcane bagasse [57,101],  corn stover [130],
corn stover [130],  brewery’s spent grain [119],
brewery’s spent grain [119],  rice straw [117],
rice straw [117],  Eucalyptus grandis [62], and
Eucalyptus grandis [62], and  rape straw [106] hydrolysates. The number included in the x axis is related to the reference number. F: Furans.
rape straw [106] hydrolysates. The number included in the x axis is related to the reference number. F: Furans.
 Picea abies [98],
Picea abies [98],  sugarcane bagasse [57,101],
sugarcane bagasse [57,101],  corn stover [130],
corn stover [130],  brewery’s spent grain [119],
brewery’s spent grain [119],  rice straw [117],
rice straw [117],  Eucalyptus grandis [62], and
Eucalyptus grandis [62], and  rape straw [106] hydrolysates. The number included in the x axis is related to the reference number.
rape straw [106] hydrolysates. The number included in the x axis is related to the reference number.
 Picea abies [98],
Picea abies [98],  sugarcane bagasse [57,101],
sugarcane bagasse [57,101],  corn stover [130],
corn stover [130],  brewery’s spent grain [119],
brewery’s spent grain [119],  rice straw [117],
rice straw [117],  Eucalyptus grandis [62], and
Eucalyptus grandis [62], and  rape straw [106] hydrolysates. The number included in the x axis is related to the reference number.
rape straw [106] hydrolysates. The number included in the x axis is related to the reference number.
 olive residues [118],
olive residues [118],  hardwood [133,134],
hardwood [133,134],  corn stover [132], and
corn stover [132], and  synthetic [131,135] hydrolysates. The number included in the x axis is related to the reference number. For: Formic acid, Lev: Levulinic acid.
synthetic [131,135] hydrolysates. The number included in the x axis is related to the reference number. For: Formic acid, Lev: Levulinic acid.
 olive residues [118],
olive residues [118],  hardwood [133,134],
hardwood [133,134],  corn stover [132], and
corn stover [132], and  synthetic [131,135] hydrolysates. The number included in the x axis is related to the reference number. For: Formic acid, Lev: Levulinic acid.
synthetic [131,135] hydrolysates. The number included in the x axis is related to the reference number. For: Formic acid, Lev: Levulinic acid.
 olive residues [142],
olive residues [142],  synthetic [17,52,141,143,144],
synthetic [17,52,141,143,144],  rice straw [117] hydrolysates, and
rice straw [117] hydrolysates, and  black liquor [145]. The number included in the x axis is related to the reference number. Lev: Levulinic acid, Lig.: lignin, Ma: Mannose, Ar: Arabinose, Ga: Galactose.
black liquor [145]. The number included in the x axis is related to the reference number. Lev: Levulinic acid, Lig.: lignin, Ma: Mannose, Ar: Arabinose, Ga: Galactose.
 olive residues [142],
olive residues [142],  synthetic [17,52,141,143,144],
synthetic [17,52,141,143,144],  rice straw [117] hydrolysates, and
rice straw [117] hydrolysates, and  black liquor [145]. The number included in the x axis is related to the reference number. Lev: Levulinic acid, Lig.: lignin, Ma: Mannose, Ar: Arabinose, Ga: Galactose.
black liquor [145]. The number included in the x axis is related to the reference number. Lev: Levulinic acid, Lig.: lignin, Ma: Mannose, Ar: Arabinose, Ga: Galactose.
 eucalyptus wood [50,147],
eucalyptus wood [50,147],  ponderosa pine wood [43], and
ponderosa pine wood [43], and  rice straw [117] hydrolysates.
rice straw [117] hydrolysates.
| Method | Characteristics | Inhibitors | Advantages | Disadvantages |
|---|---|---|---|---|
| Vacuum evaporation |
| Acids and furans |
|
|
| Liming and overliming |
| Levulinic acid, furans |
|
|
| Adsorption |
| Levulinic acid, furans and phenolics |
|
|
| Ion exchange resins |
| Acids, furans, phenolics, heavy metals |
|
|
| Liquid–liquid extraction |
| Acids, furans, and phenolics |
|
|
| Filtration by membranes operations |
| Lignin compounds |
|
|
| Raw Material | Treatment | Inhibitor | Removal (%) | Initial Concentration | Ref. |
|---|---|---|---|---|---|
| Hydrolysates from SO2-pretreated spruce wood | Activated charcoal | Furans | 94 | 1 g/L | [172] |
| Acetic acid | 28 | 1.72 g/L | |||
| Formic acid | 39 | 0.18 g/L | |||
| Phenolics | 88 | 1.3 g/L | |||
| Overliming | Furans | 45 | 1 g/L | ||
| Aliphatic acids | – | – | |||
| Phenolics | 14 | 1.3 g/L | |||
| NH4OH | Furans | 15 | 1 g/L | ||
| Aliphatic acids | – | – | |||
| Phenolics | 8 | 1.3 g/L | |||
| NaOH | Furans | 8 | 1 g/L | ||
| Aliphatic acids | 6 | 1.9 g/L | |||
| Phenolics | 1 | 1.3 g/L | |||
| Anion exchanger at pH 10 | Furans | 26 | 1 g/L | ||
| Aliphatic acids | 23 | 1.9 g/L | |||
| Phenolics | 79 | 1.3 g/L | |||
| Anion exchanger at pH 5.5 | Furans | 9 | 1 g/L | ||
| Aliphatic acids | 28 | 1.9 g/L | |||
| Phenolics | 53 | 1.3 g/L | |||
| Cation exchanger at pH 10 | Furans | 15 | 1 g/L | ||
| Aliphatic acids | 10 | 1.9 g/L | |||
| Phenolics | 22 | 1.3 g/L | |||
| Cation exchanger at pH 5.5 | Furans | 6 | 1 g/L | ||
| Aliphatic acids | 9 | 1.9 g/L | |||
| Phenolics | 8 | 1.3 g/L | |||
| Spent sulphite liquor | Nanofiltration | Lignosulphonates | 99 | 84 g/L | [174] |
| Glucose | 85 | 9.31 g/L | |||
| Xylose | 78 | 30.9 g/L | |||
| Ultrafiltration | Lignosulphonates | 57 | 84 g/L | ||
| Sugars | 76 | 49 g/L | |||
| Reverse Osmosis | Lignosulphonates | 68 | 84 g/L | ||
| Glucose | 96 | 9.31 g/L | |||
| Xylose | 93 | 30.9 g/L | |||
| Spent sulphite liquor | Cation and anion exchange | Ca2+ | 99 | 0.05% | [175] |
| Mg2+ | 100 | 0.55% | |||
| Lignosulphonates | 99 | 12% | |||
| Acetic acid | 100 | 1% | |||
| Sugars | 28 | 5% | |||
| SPORL liquid | 10 g/L lime 30 °C pH = 10 90 min | Lignosulphonates | 11 | 50 g/L | [162] |
| Glucose | 100 | 12 g/L | |||
| Xylose | 100 | 148 g/L | |||
| 20 g/L lime 30 °C pH = 12 90 min | Lignosulphonates | 26 | 50 g/L | ||
| Glucose | 100 | 12 g/L | |||
| Xylose | 60 | 148 g/L | |||
| 90 g/L lime 30 °C pH = 12.5 90 min | Lignosulphonates | 38 | 50 g/L | ||
| Glucose | 59 | 12 g/L | |||
| Xylose | 58 | 148 g/L | |||
| 20 g/L lime 75 °C pH = 12 90 min | Lignosulphonates | 36 | 50 g/L | ||
| Glucose | 21 | 12 g/L | |||
| Xylose | 5 | 148 g/L | |||
| Spent sulphite liquor | Ultrafiltration 100 kDa | Lignosulphonates | 80 | 38% | [176] |
| Spent sulphite liquor | Ultrafiltration | Lignosulphonates | 67 | 56% | [177] |
| Sugars | 95 | 32% | |||
| Acetic acid | 36 | 6.6 g/L | |||
| Spent sulphite liquor | Ultrafiltration 15 kDa/5 kDa in series | Sugars | 89 | 23.31 g/L | [178] |
| Lignosulphonates | 65 | 41.1 g/L | |||
| Ultrafiltration 15 kDa/5 kDa/1 kDa in series | Sugars | 82 | 23.31 g/L | ||
| Lignosulphonates | 72 | 41.1 g/L | |||
| Phenolics | 76 | 1.33 g/L | |||
| Spent sulphite liquor | Anionic resin, 24 h, 30 °C, 150 rpm | Acetic acid | 10 | 11.2 g/L | [179] |
| Sugars | 12 | 35.9 g/L | |||
| Lignosulphonates | 41 | 119.5 g/L | |||
| Overliming CaO pH = 11.5, 70 °C, 15 min | Acetic acid | −19 | 11.2 g/L | ||
| Sugars | 4 | 35.9 g/L | |||
| Lignosulphonates | 43 | 119.5 g/L | |||
| SO2 | 87 | 5.5 g/L | |||
| Activated carbon, 24 h, 30 °C, 150 rpm | Acetic acid | 50 | 11.2 g/L | ||
| Sugars | 6 | 35.9 g/L | |||
| Lignosulphonates | 20 | 119.5 g/L | |||
| SO2 | −13 | 5.5 g/L | |||
| Combined: CaO + resin PA408 | Acetic acid | 40 | 11.2 g/L | ||
| Sugars | 91 | 35.9 g/L | |||
| Lignosulphonates | 90 | 119.5 g/L | |||
| Combined: CaO + neutralisation with CO2 + resin | Acetic acid | −19 | 11.2 g/L | ||
| Sugars | 4 | 35.9 g/L | |||
| Lignosulphonates | 81 | 119.5 g/L | |||
| SEW Softwood | Evaporation | Sugars | 26 | 18.9% | [146] |
| Steam Stripping | +1 * | ||||
| Lime | +2 * | ||||
| Catalytic Oxidation | +2 * | ||||
| Evaporation | Lignin | 75 | 16.7% | ||
| Steam Stripping | +1 * | ||||
| Lime | +8 * | ||||
| Catalytic Oxidation | +1 * | ||||
| Evaporation | Furfural | 99 | 0.1% | ||
| Evaporation | HMF | 0 | 0.1 g/L | ||
| Evaporation | Acetic acid | −100 | 0.3% | ||
| Steam Stripping | +100 * | ||||
| SEW Spruce | Evaporation | Sugars | 6 | 17.1% | |
| Steam Stripping | +2 * | ||||
| Lime | +2 * | ||||
| Catalytic Oxidation | +0 * | ||||
| Evaporation | Lignin | 77 | 17.8% | ||
| Steam Stripping | +2 * | ||||
| Lime | +0 * | ||||
| Catalytic Oxidation | +4 * | ||||
| Evaporation | Furfural | 99 | 0.2% | ||
| Evaporation | HMF | 0 * | 0.1% | ||
| Evaporation | Acetic acid | 60 | 1.0% | ||
| Steam Stripping | +40 * | ||||
| Spent sulphite liquor | Liquid–liquid extraction with diethyl ether | Sugars | 99 | 193 g/L | [108] |
| Phenolics | 49 | 12.40 g/L | |||
| Acetic acid | 58 | 6.93 g/L | |||
| Levulinic acid | 64 | 0.11 g/L | |||
| Formic acid | 94 | 0.23 g/L | |||
| Furfural | 88 | 0.20 g/L | |||
| HMF | 81 | 0.13 g/L | |||
| Liquid–liquid extraction with chloroform | Sugars | 99 | 193 g/L | ||
| Phenolics | 56 | 12.40 g/L | |||
| Acetic acid | 75 | 6.93 g/L | |||
| Levulinic acid | 75 | 0.11 g/L | |||
| Formic acid | 97 | 0.23 g/L | |||
| Furfural | 97 | 0.20 g/L | |||
| HMF | 92 | 0.13 g/L | |||
| Spent sulphite liquor | Ethyl acetate pH 3.4 | Phenolics | 89–100 | 12.4 g/L | [180] |
| Ethyl acetate pH 2 | Phenolics | 67–73 | 12.4 g/L |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coz, A.; Llano, T.; Cifrián, E.; Viguri, J.; Maican, E.; Sixta, H. Physico-Chemical Alternatives in Lignocellulosic Materials in Relation to the Kind of Component for Fermenting Purposes. Materials 2016, 9, 574. https://doi.org/10.3390/ma9070574
Coz A, Llano T, Cifrián E, Viguri J, Maican E, Sixta H. Physico-Chemical Alternatives in Lignocellulosic Materials in Relation to the Kind of Component for Fermenting Purposes. Materials. 2016; 9(7):574. https://doi.org/10.3390/ma9070574
Chicago/Turabian StyleCoz, Alberto, Tamara Llano, Eva Cifrián, Javier Viguri, Edmond Maican, and Herbert Sixta. 2016. "Physico-Chemical Alternatives in Lignocellulosic Materials in Relation to the Kind of Component for Fermenting Purposes" Materials 9, no. 7: 574. https://doi.org/10.3390/ma9070574
APA StyleCoz, A., Llano, T., Cifrián, E., Viguri, J., Maican, E., & Sixta, H. (2016). Physico-Chemical Alternatives in Lignocellulosic Materials in Relation to the Kind of Component for Fermenting Purposes. Materials, 9(7), 574. https://doi.org/10.3390/ma9070574