Adsorption of Heavy Metals by Graphene Oxide/Cellulose Hydrogel Prepared from NaOH/Urea Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
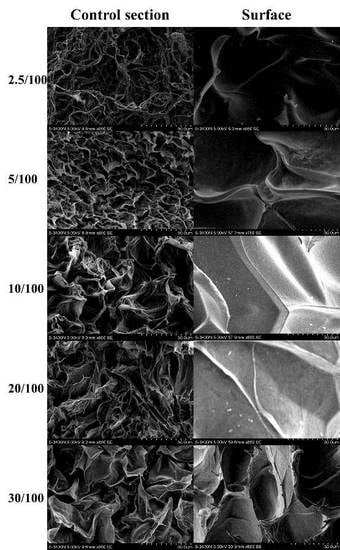
2.1. Characterization of GO/Cellulose Hydrogel
2.2. Adsorption Measurements
2.2.1. Effect of GO/Celluloses Ratios, Cu(II) Solution pH, and Dosage on Cu2+ Uptake
2.2.2. Adsorption Kinetics Studies
2.2.3. Adsorption Isotherm Studies
2.2.4. Repeated Use of Hydrogel and Adsorption of Other Hazardous Metals
3. Materials and Methods
3.1. Materials
3.2. Preparation of GO
3.3. Preparation of GO/Cellulose Hydrogel
3.4. Characterization
3.5. Adsorption Studies
3.5.1. Preparation of Cu2+ Solution
3.5.2. Adsorption Procedures
3.5.3. Desorption and Reusability Behaviors of GO/Cellulose Hydrogel
3.5.4. Adsorption of Other Hazardous Metals
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peng, X.W.; Zhong, L.X.; Ren, J.L.; Sun, R.C. Highly effective adsorption of heavy metal ions from aqueous solutions by macroporous xylan-rich hemicelluloses-based hydrogel. J. Agric. Food Chem. 2012, 60, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Ogata, F.; Kangawa, M.; Iwata, Y.; Ueda, A.; Tanaka, Y.; Kawasaki, N. A Study on the adsorption of heavy metals by using raw wheat bran bioadsorbent in aqueous solution phase. Chem. Pharm. Bull. 2014, 62, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Luo, C.; Li, X.; Lu, F.; Qiu, H.; Sun, M. Fabrication of novel magnetic chitosan grafted with graphene oxide to enhance adsorption properties for methyl blue. J. Hazard. Mater. 2012, 215, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.X.; Peng, X.W.; Yang, D.; Sun, R.C. Adsorption of heavy metals by a porous bioadsorbent from lignocellulosic biomass reconstructed in an ionic liquid. J. Agric. Food Chem. 2012, 60, 5621–5628. [Google Scholar] [CrossRef] [PubMed]
- El-Hag Ali, A.; Shawky, H.A.; Abd El Rehim, H.A.; Hegazy, E.A. Synthesis and characterization of PVP/AAc copolymer hydrogel and its applications in the removal of heavy metals from aqueous solution. Eur. Polym. J. 2003, 39, 2337–2344. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; Zhang, L. Structure and properties of β-cyclodextrin/cellulose hydrogels prepared in NaOH/urea aqueous solution. Carbohyd. Polym. 2013, 94, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohyd. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Li, R.; Wang, S.; Lu, A.; Zhang, L. Dissolution of cellulose from different sources in an NaOH/urea aqueous system at low temperature. Cellulose 2015, 22, 339–349. [Google Scholar] [CrossRef]
- Fan, J.; Shi, Z.; Lian, M.; Li, H.; Yin, J. Mechanically strong graphene oxide/sodium alginate/polyacrylamide nanocomposite hydrogel with improved dye adsorption capacity. J. Mater. Chem. A 2013, 1, 7433–7443. [Google Scholar] [CrossRef]
- Madadrang, C.J.; Kim, H.Y.; Gao, G.; Wang, N.; Zhu, J.; Feng, H.; Gorring, M.; Kasner, M.L.; Hou, S. Adsorption behavior of EDTA-graphene oxide for Pb(II) removal. ACS Appl. Mater. Interfaces 2012, 4, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wen, T.; Wu, X.; Chen, C.; Hu, J.; Li, J.; Wang, X. Synthesis of porous Fe3O4 hollow microspheres/graphene oxide composite for Cr(VI) removal. Dalton Trans. 2013, 42, 14710–14717. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Mu, L.; Wen, J.; Zhou, Q. Immobilized smart RNA on graphene oxide nanosheets to specifically recognize and adsorb trace peptide toxins in drinking water. J. Hazard. Mater. 2012, 213, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Tao, X.; Yang, Z.; Li, K.; Yang, H.; Li, A.; Cheng, R. Effects of the oxidation degree of graphene oxide on the adsorption of methylene blue. J. Hazard. Mater. 2014, 268, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Wang, X.; Sun, Z.; Ma, J.; Wu, T.; Xing, F.; Gao, J. Porous graphene oxide/carboxymethyl cellulose monoliths, with high metal ion adsorption. Carbohyd. Polym. 2014, 101, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, Y.; Feng, J.; Wu, P. Graphene-oxide-sheet-induced gelation of cellulose and promoted mechanical properties of composite aerogels. J. Phys. Chem. C 2012, 116, 8063–8068. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, R.Z.; Ma, Y.Q.; Guan, W.B.; Wu, X.L.; Liu, X.; Li, H.; Du, Y.L.; Pan, C.P. Preparation of cellulose/graphene composite and its applications for triazine pesticides adsorption from water. ACS Sustain. Chem. Eng. 2015, 3, 396–405. [Google Scholar] [CrossRef]
- Liu, J.; Yang, T.; Chen, S.; Chen, X.; Wang, J. Nickel chelating functionalization of graphene composite for metal affinity membrane isolation of lysozyme. J. Mater. Chem. B 2013, 1, 810–818. [Google Scholar] [CrossRef]
- Belfer, S.; Fainchtain, R.; Purinson, Y.; Kedem, O. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes unmodified modified and protein fouled. J. Membr. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Garg, U.; Kaur, M.P.; Jawa, G.K.; Sud, D.; Garg, V.K. Removal of cadmium (II) from aqueous solutions by adsorption on agricultural waste biomass. J. Hazard. Mater. 2008, 154, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, C.; Li, D.; Chen, Y.; Zhong, G.; Li, Z. Ultra low gas permeability and efficient reinforcement of cellulose nanocomposite films by well aligned graphene oxide nanosheets. J. Mater. Chem. A 2014, 2, 15853–15863. [Google Scholar] [CrossRef]
- Sun, G.; Li, X.; Qu, Y.; Wang, X.; Yan, H.; Zhang, Y. Preparation and characterization of graphite nanosheets from detonation technique. Mater. Lett. 2008, 62, 703–706. [Google Scholar] [CrossRef]
- Han, D.; Yan, L.; Chen, W.; Li, W.; Bangal, P.R. Cellulose/graphite oxide composite films with improved mechanical properties over a wide range of temperature. Carbohyd. Polym. 2011, 83, 966–972. [Google Scholar] [CrossRef]
- Öztürk, H.B.; Potthast, A.; Rosenau, T.; Abu-Rous, M.; MacNaughtan, B.; Schuster, K.C.; Mitchell, J.R.; Bechtold, T. Changes in the intra- and inter-fibrillar structure of lyocell (TENCEL®) fibers caused by NaOH treatment. Cellulose 2008, 16, 37–52. [Google Scholar] [CrossRef]
- Ouyang, W.; Sun, J.; Memon, J.; Wang, C.; Geng, J.; Huang, Y. Scalable preparation of three-dimensional porous structures of reduced graphene oxide/cellulose composites and their application in supercapacitors. Carbon 2013, 62, 501–509. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Zhang, L. Fabrication and characterization of novel macroporous cellulose–alginate hydrogels. Polymer 2009, 50, 5467–5473. [Google Scholar] [CrossRef]
- Chang, C.; Han, K.; Zhang, L. Structure and properties of cellulose/poly(N-isopropylacrylamide) hydrogels prepared by IPN strategy. Polym. Adv. Technol. 2009, 22, 1329–1334. [Google Scholar] [CrossRef]
- Yu, X.; Kang, D.; Hu, Y.; Tong, S.; Ge, M.; Caob, C.; Songb, W. One-pot synthesis of porous magnetic cellulose beads for the removal of metal ions. RSC Adv. 2014, 4, 31362–31369. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Vinu, A.; Murugesan, V.; Hartmann, M. Adsorption of lysozyme over mesoporous molecular sieves MCM-41 and SBA-15: Influence of pH and aluminum incorporation. J. Phys. Chem. B 2004, 108, 7323–7330. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Sheng, G.; Hu, J.; Tan, X.; Wang, X. Effect of surfactants on Pb(II) adsorption from aqueous solutions using oxidized multiwall carbon nanotubes. Chem. Eng. J. 2011, 166, 551–558. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Y.; Zhou, H.; Ye, T.; Huang, Z.; Liu, R.; Kuang, Y. Highly efficient removal of Cu(II) from aqueous solution by using graphene oxide. Water Air Soil Pollut. 2012, 224, 1–8. [Google Scholar] [CrossRef]
- Edokpayi, J.; Odiyo, J.; Popoola, E.; Alayande, O.; Msagati, T. Synthesis and characterization of biopolymeric chitosan derived from land snail shells and its potential for Pb2+ removal from aqueous solution. Materials 2015, 8, 8630–8640. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, H.; Ma, J.W. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—Bamboo charcoal. J. Hazard. Mater. 2010, 177, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Kumar, E.; Bhatnagar, A.; Ji, M.; Jung, W.; Lee, S.H.; Kim, S.J.; Lee, G.; Song, H.; Choi, J.Y.; Yang, J.S.; et al. Defluoridation from aqueous solutions by granular ferric hydroxide (GFH). Water Res. 2009, 43, 490–498. [Google Scholar] [CrossRef] [PubMed]
- OuYang, X.K.; Jin, R.N.; Yang, L.P.; Wen, Z.S.; Yang, L.Y.; Wang, Y.G.; Wang, C.Y. Partially hydrolyzed bamboo (Phyllostachys heterocycla) as a porous bioadsorbent for the removal of Pb(II) from aqueous mixtures. J. Agric. Food Chem. 2014, 62, 6007–6015. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Chen, X.; Kim, U.J.; Kimura, S.; Wada, M.; Saito, T.; Isogai, A. TEMPO-oxidized cellulose hydrogel as a high-capacity and reusable heavy metal ion adsorbent. J. Hazard. Mater. 2013, 260, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, J.; Cheng, L.; Lu, C.; Wang, Y.; He, X.; Zhang, W. Acrylic acid grafted and acrylic acid/sodium humate grafted bamboo cellulose nanofi bers for Cu2+ adsorption. RSC Adv. 2014, 4, 55195–55201. [Google Scholar] [CrossRef]
- Peng, S.; Meng, H.; Ouyang, Y.; Chang, J. Nanoporous magnetic cellulose–chitosan composite microspheres: Preparation, characterization, and application for Cu(II) adsorption. Ind. Eng. Chem. Res. 2014, 53, 2106–2113. [Google Scholar] [CrossRef]
- Tan, I.A.; Ahmad, A.L.; Hameed, B.H. Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Qu, J.; Chen, Y. Magnetic powder MnO-Fe2O3 composite—A novel material for the removal of azo-dye from water. Water Res. 2005, 39, 630–638. [Google Scholar] [CrossRef] [PubMed]
- William, S.; Hummers, J.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar]
- Chen, X.; Chen, J.; You, T.; Wang, K.; Xu, F. Effects of polymorphs on dissolution of cellulose in NaOH/urea aqueous solution. Carbohyd. Polym. 2015, 125, 85–91. [Google Scholar] [CrossRef] [PubMed]












| Sample | 2.5 | 5 | 10 | 20 | 30 |
|---|---|---|---|---|---|
| Water content (wt %) | 92.7 | 90.3 | 94.1 | 92.5 | 93.5 |
| Compressive modulus (kPa) | 114 | 193 | 144 | 128 | 115 |
| SBET (m2·g−1) | 0.19 | 13.41 | 40.72 | 25.11 | 45.12 |
| Pore volume (cm3·g−1) | 0.0023 | 0.0575 | 0.1807 | 0.0048 | 0.1856 |
| Pore size (nm) | 3.11 | 7.49 | 7.08 | 5.15 | 6.73 |
| C0 (mg·L−1) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| (mg·g−1) | k1 (g·mg−1·min−1) | R2 | (mg·g−1) | k2 (g·mg−1·min−1) | R2 | |
| 50 | 16.44 | 0.0106 | 0.876 | 37.59 | 0.0023 | 0.991 |
| 200 | 46.92 | 0.0187 | 0.846 | 94.34 | 0.0009 | 0.998 |
| T (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qmax (mg·g−1) | b (L·mg−1) | R2 | k (mg1−1/n·L1/n·g−1) | n | R2 | |
| 293 | 138.888 | 0.014 | 0.9552 | 18.01 | 3.19 | 0.9703 |
| 298 | 384.615 | 0.001 | 0.9406 | 0.92 | 1.19 | 0.9982 |
| 303 | 192.308 | 0.004 | 0.8098 | 1.72 | 3.78 | 0.9633 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhou, S.; Zhang, L.; You, T.; Xu, F. Adsorption of Heavy Metals by Graphene Oxide/Cellulose Hydrogel Prepared from NaOH/Urea Aqueous Solution. Materials 2016, 9, 582. https://doi.org/10.3390/ma9070582
Chen X, Zhou S, Zhang L, You T, Xu F. Adsorption of Heavy Metals by Graphene Oxide/Cellulose Hydrogel Prepared from NaOH/Urea Aqueous Solution. Materials. 2016; 9(7):582. https://doi.org/10.3390/ma9070582
Chicago/Turabian StyleChen, Xiong, Sukun Zhou, Liming Zhang, Tingting You, and Feng Xu. 2016. "Adsorption of Heavy Metals by Graphene Oxide/Cellulose Hydrogel Prepared from NaOH/Urea Aqueous Solution" Materials 9, no. 7: 582. https://doi.org/10.3390/ma9070582
APA StyleChen, X., Zhou, S., Zhang, L., You, T., & Xu, F. (2016). Adsorption of Heavy Metals by Graphene Oxide/Cellulose Hydrogel Prepared from NaOH/Urea Aqueous Solution. Materials, 9(7), 582. https://doi.org/10.3390/ma9070582