Optimization of Layered Cathode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis Procedures
2.2. Characterizations
2.3. Electrochemical Tests
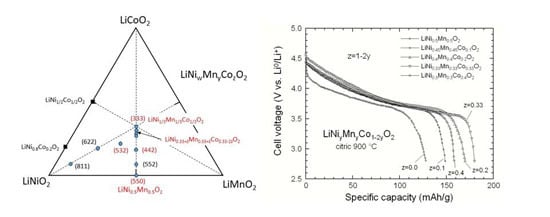
3. Results and Discussion
3.1. The Effect of Particle Size
3.2. The Effect of Cationic Mixing
3.3. Adjustment of the Li/M Ratio
3.4. Effect of the Chelating Agent
3.5. Effect of the Synthesis Recipe
3.6. Effect of Small Deviation of Co Content
3.7. Layered-Layered Integrated Materials
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Julien, C.M.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries: Science and Technology; Springer: Heidelberg, Germany, 2015; p. 630. [Google Scholar]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative issues of cathode materials for Li-ion batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef]
- Zaghib, K.; Mauger, A.; Groult, H.; Goodenough, J.B.; Julien, C.M. Advanced electrode of high-power Li-ion batteries. Materials 2013, 6, 1028–1049. [Google Scholar] [CrossRef]
- Fergus, J.W. Recent developments in cathode materials for lithium ion batteries. J. Power Sources 2010, 195, 939–954. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M. Nanoscience supporting the research on the negative electrodes of Li-ion batteries. Nanomaterials 2015, 5, 2279–2301. [Google Scholar] [CrossRef]
- Vediappan, K.; Guerfi, A.; Gariépy, V.; Demopoulos, G.P.; Hovington, P.; Trottier, J.; Mauger, A.; Zaghib, K.; Julien, C.M. Stirring effect in hydrothermal synthesis of C-LiFePO4. J. Power Sources 2014, 266, 99–106. [Google Scholar] [CrossRef]
- Hovington, P.; Lagacé, M.; Guerfi, A.; Bouchard, P.; Mauger, A.; Julien, C.M.; Armand, M.; Zaghib, K. New lithium metal polymer solid state battery for an ultrahigh energy: Nano C-LiFePO4 versus nano Li1.2V3O8. Nano Lett. 2015, 15, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, A.; Lee, J.Y. Synthesis and characterization of LiNi1-x-yCoxMnyO2 as the cathode materials of secondary lithium batteries. J. Power Sources 1999, 81–82, 416–419. [Google Scholar] [CrossRef]
- Ohzuku, T.; Makimura, Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries. Chem. Mater. 2001, 30, 642–643. [Google Scholar] [CrossRef]
- MacNeil, D.D.; Lu, Z.; Dahn, J.R. Structure and electrochemistry of Li(NixCo1-2xMnx)O2 (0 ≤ x ≤ 1/2). J. Electrochem. Soc. 2002, 149, A1332–A1336. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Ohzuku, T. Novel lithium insertion material of Li(Co1/3Ni1/3Mn1/3)O2 for advanced lithium-ion batteries. J. Power Sources 2003, 119, 171–174. [Google Scholar] [CrossRef]
- Belharouak, I.; Sun, Y.K.; Liu, J.; Amine, K. Li(Ni1/3Co1/3Mn1/3)O2 as a suitable cathode for high power applications. J. Power Sources 2003, 123, 247–252. [Google Scholar] [CrossRef]
- Zhang, X.; Mauger, A.; Lu, Q.; Groult, H.; Perrigaud, L.; Gendron, F.; Julien, C.M. Synthesis and characterization of LiNi1/3Mn1/3Co1/3O2 by wet-chemical method. Electrochim. Acta 2010, 55, 6440–6449. [Google Scholar] [CrossRef]
- Shaju, K.M.; Subba Rao, G.V.; Chowdari, B.V.R. Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries. Electrochim. Acta 2002, 48, 145–151. [Google Scholar] [CrossRef]
- Kim, J.M.; Chung, H.T. The first cycle characteristics of Li(Ni1/3Mn1/3Co1/3)O2 charged up to 4.7 V. Electrochim. Acta 2004, 49, 937–944. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Song, C.H.; Stephan, A.M.; Kim, A.; Nahm, K.S. Influence of solvents on the structural and electrochemical properties of Li(Li0.2Ni0.1Co0.2Mn0.5)O2 prepared by a solvothermal reaction method. J. Electrochem. Soc. 2006, 153, A390–A395. [Google Scholar] [CrossRef]
- Julien, C.; El-Farh, L.; Rangan, S.; Massot, M. Studies of LiNi0.6Co0.4O2 cathode material prepared by the citric acid-assisted sol-gel method for lithium batteries. J. Sol-Gel Sci. Technol. 1999, 15, 63–72. [Google Scholar] [CrossRef]
- Julien, C.; Letranchant, C.; Rangan, S.; Lemal, M.; Ziolkiewicz, S.; Castro-Garcia, S.; El-Farh, L.; Benkaddour, M. Layered LiNi0.5Co0.5O2 cathode materials grown by soft-chemistry via various solution methods. Mater. Sci. Eng. B 2000, 76, 145–155. [Google Scholar] [CrossRef]
- El-Farh, L.; Massot, M.; Lemal, M.; Julien, C. Physical properties and electrochemical features of lithium nickel-cobalt oxide cathode materials prepared at moderate temperature. J. Electroceram. 1999, 3, 425–432. [Google Scholar] [CrossRef]
- Julien, C.; Nazri, G.A.; Rougier, A. Electrochemical performances of layered LiM1-yM’yO2 (M = Ni, Co; M′ = Mg, Al, B) oxides in lithium batteries. Solid State Ion. 2000, 135, 121–130. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Whittingham, M.S. Hydrothermal synthesis of cathode materials. J. Power Sources 2007, 174, 442–448. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.J.; Zhu, X.P.; Mauger, A.; Lu, Q.; Julien, C.M. Aging of LiNi1/3Mn1/3Co1/3O2 cathode material upon exposure to H2O. J. Power Sources 2011, 196, 5102–5108. [Google Scholar] [CrossRef]
- Abdel-Ghany, A.; Zaghib, K.; Gendron, F.; Mauger, A.; Julien, C.M. Structural, magnetic and electrochemical properties of LiNi0.5Mn0.5O2 as positive electrode for Li-ion batteries. Electrochim. Acta 2007, 52, 4092–4100. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.C.; Hinokuma, K. Optimized LiFePO4 for lithium battery cathode. J. Electrochem. Soc. 2001, 148, A224–A229. [Google Scholar] [CrossRef]
- Okubo, M.; Hosono, E.; Kim, J.; Enomoto, M.; Kojima, N.; Kudo, T.; Zhou, H.; Honma, I. Nanosize effect on high-rate Li-ion intercalation in LiCoO2 electrode. J. Am. Chem. Soc. 2007, 129, 7444–7452. [Google Scholar] [CrossRef] [PubMed]
- Castro-Couceiro, A.; Castro-Garcia, S.; Senaris-Rodriguez, M.A.; Soulette, F.; Julien, C. Effects of the aluminum doping on the microstructure and morphology of LiNi0.5Co0.5O2 oxides. Ionics 2002, 8, 192–200. [Google Scholar] [CrossRef]
- Brochu, F.; Guerfi, A.; Trottier, J.; Kopec, M.; Mauger, A.; Groult, H.; Julien, C.M.; Zaghib, K. Structure and electrochemistry of scaling nano C-LiFePO4 synthesized by hydrothermal route: Complexing agent effect. J. Power Sources 2012, 214, 1–6. [Google Scholar] [CrossRef]
- Lee, K.S.; Myung, S.T.; Prakash, J.; Yashiro, H.; Sun, Y.K. Optimization of microwave synthesis of Li(Ni0.4Co0.2Mn0.4)O2 as a positive electrode material for lithium batteries. Electrochim. Acta 2008, 53, 3065–3074. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.J.; Mauger, A.; Li, Q.; Gendron, F.; Julien, C.M. Minimization of the cation mixing in Li1+x(NMC)1-xO2 as cathode material. J. Power Sources 2010, 195, 1292–1301. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Chao, D.; Baikie, T.; Bai, L.; Chen, S.; Zhao, Y.; Sum, T.C.; Lin, J.; Shen, Z. Hierarchical porous LiNi1/3Co1/3Mn1/3O2 nano-micro spherical cathode material: Minimizes cation mixing and improved Li+ mobility for enhanced electrochemical performance. Sci. Rep. 2016, 6, 25771. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.Y.; Duh, J.G.; Sheen, S.R. Effect on Mn content on the microstructure and electrochemical performance of LiNi0.75-xCo0.25MnxO2 cathode materials. J. Electrochem. Soc. 2005, 152, A1695–A1700. [Google Scholar] [CrossRef]
- Kang, K.; Meng, Y.S.; Bréger, J.; Grey, C.P.; Ceder, G. Electrodes with high power and high capacity for rechargeable lithium batteries. Science 2006, 311, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Park, S.H.; Park, C.W.; Sun, Y.K. Structural and electrochemical properties of layered Li(Ni0.5Mn0.5)1-xCoxO2 positive materials synthesized by ultrasonic spray pyrolysis method. Solid State Ion. 2004, 171, 167–172. [Google Scholar] [CrossRef]
- Tran, N.; Croguenec, L.; Jordy, C.; Biensan, P.; Delmas, C. Influence of the synthesis route on the electrochemical properties of LiNi0.425Mn0.425Co0.15O2. Solid State Ion. 2005, 176, 1539–1547. [Google Scholar] [CrossRef]
- Ngala, J.K.; Chernova, N.A.; Ma, M.; Mamak, M.; Zavalij, P.Y.; Whittingham, M.S. The synthesis, characterization and electrochemical behavior of the layered LiNi0.4Mn0.4Co0.2O2 compound. J. Mater. Chem. 2004, 14, 214–220. [Google Scholar] [CrossRef]
- Tsai, Y.W.; Lee, J.F.; Liu, D.G.; Hwang, B.J. In-situ X-ray absorption spectroscopy investigations of a layered LiNi0.65Co0.25Mn0.1O2 cathode material for rechargeable lithium batteries. J. Mater. Chem. 2004, 14, 958–965. [Google Scholar] [CrossRef]
- Mauger, A.; Gendron, F.; Julien, C.M. Magnetic properties of LixNiyMnyCo1−2yO2 (0.2 ≤ 1 − 2y ≤ 0.5, 0 ≤ x ≤ 1). J. Alloys Compd. 2012, 520, 42–51. [Google Scholar] [CrossRef]
- Julien, C.M.; Ait-Salah, A.; Mauger, A.; Gendron, F. Magnetic properties of intercalation compounds as positive electrodes for Li-ion batteries. Ionics 2006, 12, 21–32. [Google Scholar] [CrossRef]
- Samarasinghab, P.; Tran-Nguyen, D.H.; Behma, M.; Wijayasinghe, A. LiNi1/3Mn1/3Co1/3O2 synthesized by the Pechini method for the positive electrode in Li-ion batteries: Material characteristics and electrochemical behaviour. Electrochim. Acta 2008, 53, 7995–8000. [Google Scholar] [CrossRef]
- Myung, S.T.; Komaba, S.; Kurihara, K.; Hosoya, K.; Kumagai, N.; Sun, Y.K.; Nakai, I.; Yonemura, M.; Kamiyama, T. Synthesis of Li((Ni0.5Mn0.5)1-xLix)O2 by emulsion drying method and impact of excess Li on structural and electrochemical properties. Chem. Mater. 2006, 18, 1658–1666. [Google Scholar] [CrossRef]
- Xu, B.; Qian, D.; Wang, Z.; Meng, Y.S. Recent progress in cathode materials research for advanced lithium ion batteries. Mater. Sci. Eng. R 2012, 73, 51–65. [Google Scholar] [CrossRef]
- Shaju, K.M.; Subba Rao, G.V.; Chowdari, B.V.R. Li-ion kinetics and polarization effect on the electrochemical performance of Li(Ni1/2Mn1/2)O2. Electrochim. Acta 2004, 49, 1565–1576. [Google Scholar] [CrossRef]
- Julien, C.; Michael, S.S.; Ziolkiewicz, S. Structural and electrochemical properties of LiNi0.3Co0.7O2 synthesized by different low-temperature techniques. Intl. J. Inorg. Mater. 1999, 1, 29–37. [Google Scholar] [CrossRef]
- Ben-Kamel, K.; Ben-Amor, G.; Amdouni, N.; Mauger, A.; Groult, H.; Julien, C.M. LiNi0.33+δMn0.33+δCo0.33–2δO2 (0.025 ≤ δ ≤ 0.075) cathode materials for Li-ion batteries: Local structure. ECS Trans. 2011, 35, 129–134. [Google Scholar]
- Ben Kamel, K.; Amdouni, N.; Trottier, J.; Zaghib, K.; Mauger, A.; Groult, H.; Julien, C.M. LiNi0.33+δMn0.33+δCo0.33–2δO2 (0.0 ≤ δ ≤ 0.075) cathode materials for Li-ion batteries: Electrochemical features. ECS Trans. 2011, 35, 135–139. [Google Scholar]
- Ye, D.L.; Wang, L.Z. Li2MnO3 based Li-rich cathode materials: Towards a better tomorrow of high energy lithium ion batteries. Mater. Technol. Adv. Perform. Mater. 2014, 29, A59–A69. [Google Scholar]
- Yu, H.; Zhou, H. High-energy cathode materials (Li2MnO3−LiMO2) for lithium-ion batteries. J. Phys. Chem. Lett. 2013, 4, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Dahn, J.R. Structure and electrochemistry of layered Li(CrxLi(1/3-x/3)Mn(2/3-2x/3)O2. J. Electrochem. Soc. 2002, 149, A1454–A1459. [Google Scholar] [CrossRef]
- Deng, Z.; Manthiram, A. Influence of cationic substitutions on the oxygen loss and reversible capacity of lithium-rich layered oxide cathodes. J. Phys. Chem. C 2011, 115, 7097–7103. [Google Scholar] [CrossRef]
- Muhammad, S.; Kim, H.; Kim, Y.; Kim, D.; Song, J.H.; Yoon, J.; Park, J.H.; Ahn, S.J.; Kang, S.H.; Thakeray, M.M.; et al. Evidence of reversible oxygen participation in anomalously high capacity Li- and Mn-rich cathodes for Li-ion batteries. Nano Energy 2016, 21, 172–184. [Google Scholar] [CrossRef]
- Lee, D.K.; Park, S.H.; Amine, K.; Bang, H.J.; Parakash, J.; Sun, Y.K. High capacity Li(Li0.2Ni0.2Mn0.6)O2 cathode materials via a carbonate co-precipitation method. J. Power Sources 2006, 162, 1346–1350. [Google Scholar] [CrossRef]
- Liu, Y.J.; Liu, S.B.; Wang, Y.P.; Chen, L.; Chen, X.H. Effect of MnO2 modification on electrochemical performance of LiNi0.2Li0.2Mn0.6O2 layered solid solution cathode. J. Power Sources 2013, 222, 455–460. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, M.; Hu, X.; Zhao, Z.; Wang, Y.; Lv, Z. Effects of Sn doping on the structural and electrochemical properties of Li1.2Ni0.2Mn0.6O2 Li-rich cathode materials. Electrochim. Acta 2015, 174, 1167–1174. [Google Scholar] [CrossRef]
- Lu, Z.; MacNeil, D.; Dahn, J.R. Layered cathode materials Li(NixLi(1/3–2x/3)Mn(2/3-x/3))O2 for lithium-ion batteries. Electrochem. Solid-State Lett. 2001, 4, A191–A194. [Google Scholar] [CrossRef]
- Lu, Z.; Dahn, J.R. Understanding the anomalous capacity of Li(NixLi(1/3–2x/3)Mn(2/3-x/3))O2 cells using in situ X-ray diffraction and electrochemical studies. J. Electrochem. Soc. 2002, 149, A815–A822. [Google Scholar] [CrossRef]
- Lu, Z.; Beaulieu, L.; Donaberger, R.; Thomas, C.; Dahn, J.R. Synthesis, structure, and electrochemical behavior of Li(NixLi(1/3–2x/3)Mn(2/3-x/3))O2. J. Electrochem. Soc. 2002, 149, A778–A791. [Google Scholar] [CrossRef]
- Boulineau, A.; Simonin, L.; Colin, J.-F.; Bourbon, C.; Patoux, S. First Evidence of manganese–nickel segregation and densification upon cycling in Li-rich layered oxides for lithium batteries. Nano Lett. 2013, 13, 3857–3863. [Google Scholar] [CrossRef] [PubMed]
- McCalla, E.; Rowe, A.W.; Camardese, J.; Dahn, J.R. The role of metal site vacancies in promoting Li–Mn–Ni–O layered solid solutions. Chem. Mater. 2013, 25, 2716–2721. [Google Scholar] [CrossRef]
- Yu, H.; Ishikawa, R.; So, Y.G.; Shibata, N.; Kudo, T.; Zhou, H.; Ikuhara, Y. Direct atomic-resolution observation of two phases in the Li1.2Mn0.567Ni0.166Co0.067O2 cathode material for lithium-ion batteries. Angew. Chem. Int. Ed. 2013, 52, 5969–5973. [Google Scholar] [CrossRef] [PubMed]
- Hy, S.; Felix, F.; Rick, J.; Su, W.-N.; Hwang, B.J. Direct in situ observation of Li2O evolution on Li-rich high-capacity cathode material, Li(NixLi(1/3–2x/3)Mn(2/3-x/3))O2 (0 ≤ x ≤ 0.5). J. Am. Chem. Soc. 2014, 136, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, N.; Su, Y.; Shou, H.; Bao, L.; Yang, W.; Zhang, L.; An, R.; Chen, S. Spinel/layered heterostructured cathode material for high-capacity and high-rate Li-ion batteries. Adv. Mater. 2013, 25, 3722–3726. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kim, H.; Wang, Y.; He, P. High-energy ‘composite’ layered manganese-rich cathode materials via controlling Li2MnO3 phase activation for lithium-ion batteries. Phys. Chem. Chem. Phys. 2012, 14, 6584–6595. [Google Scholar] [CrossRef] [PubMed]
- Rowe, W.; Dahn, J.R. Positive Electrode Materials in the Li-Mn-Ni-O System exhibiting anomalous capacity growth during extended cycling. J. Electrochem. Soc. 2014, 161, A308–A317. [Google Scholar] [CrossRef]
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.-G.; et al. Formation of the spinel phase in the layered composite cathode used in Li-ion batteries. ACS Nano 2013, 7, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Lai, M.O.; Liu, Z.; Liu, H.; Lu, L. Graphene-based surface modification on layered Li-rich cathode for high-performance Li-ion batteries. J. Mater. Chem A 2013, 1, 9954–9965. [Google Scholar] [CrossRef]
- Song, B.; Liu, H.; Liu, Z.; Xiao, P.; Lai, M.O.; Lu, L. High rate capability caused by surface cubic spinels in Li-rich layer-structured cathodes for Li-ion batteries. Sci. Rep. 2013, 3, 3094. [Google Scholar] [CrossRef] [PubMed]























| Sample | a (Å) | c (Å) | c/a | V (Å3) | L (nm) | I003/I104 | R | η (%) |
|---|---|---|---|---|---|---|---|---|
| 550 | 2.8833(2) | 14.3251(2) | 4.948 | 119.08(9) | 59.9 | 1.197 | 0.420 | 6.70 |
| 4½4½1 | 2.8766(8) | 14.3005(5) | 4.971 | 118.33(2) | 65.5 | 1.209 | 0.418 | 5.31 |
| 532 | 2.8732(2) | 14.2684(4) | 4.966 | 102.01(2) | 61.6 | 1.252 | 0.415 | 3.34 |
| 442 | 2.8685(1) | 14.2655(3) | 4.973 | 101.65(1) | 64.6 | 1.425 | 0.401 | 2.97 |
| 333 | 2.8604(2) | 14.2376(2) | 4.977 | 100.88(2) | 72.3 | 1.456 | 0.387 | 1.56 |
| 334 | 2.8622(3) | 14.2455(7) | 4.977 | 116.70(2) | 63.8 | 1.450 | 0.395 | 1.77 |
| ¼¼½ | 2.8628(5) | 14.2259(6) | 4.981 | 116.86(6) | 62.0 | 1.452 | 0.390 | 0.70 |
| Cathode | (1 − 2y) | E0,1 (V) | −K1 | E0,2 (V) | −K2 |
|---|---|---|---|---|---|
| 550 | 0.00 | 3.737 | 3.82 | 3.869 | 3.12 |
| 4½4½1 | 0.10 | 3.734 | 3.75 | 3.903 | 2.98 |
| 442 | 0.20 | 3.731 | 3.75 | 3.955 | 2.98 |
| 333 | 0.33 | 3.722 | 3.71 | 3.932 | 3.00 |
| 334 | 0.40 | 3.717 | 3.78 | 3.921 | 3.01 |
| (1 + x) | a (Å) | c (Å) | c/a | V (Å3) | L (nm) | ηR (%) | ηM (%) |
|---|---|---|---|---|---|---|---|
| 1.02 | 2.8650(1) | 14.2252(2) | 4.975 | 101.31(2) | 59.4 | 1.98 | 1.88 |
| 1.04 | 2.8603(2) | 14.241(2) | 4.979 | 100.90(3) | 55.9 | 1.43 | 1.50 |
| Crystal Data | R = 0.5 | R = 1.0 | R = 2.0 | R = 3.0 | R = 4.0 |
|---|---|---|---|---|---|
| a (Å) | 2.8680(2) | 2.8671(2) | 2.8602(1) | 2.8675(2) | 2.8684(3) |
| c (Å) | 14.2588(3) | 14.2593(2) | 14.2458(2) | 14.2589(4) | 14.2619(3) |
| c/a | 4.9716(4) | 4.9734(4) | 4.9807(2) | 4.9726(5) | 4.9720(6) |
| V (Å 3) | 101.57(2) | 101.51(2) | 100.92(1) | 101.53(2) | 101.62(2) |
| L (Å) | ≈570 | ≈540 | ≈490 | ≈550 | ≈610 |
| I(003)/I(104) | 1.440 | 1.614 | 1.728 | 1.558 | 1.423 |
| (I(006) + I(102))/I(101) | 0.482 | 0.427 | 0.378 | 0.438 | 0.459 |
| S(MO2) (Å) | 2.126(2) | 2.120(5) | 2.109(3) | 2.123(3) | 2.126(2) |
| I(LiO2) (Å) | 2.627(2) | 2.633(6) | 2.640(3) | 2.630(3) | 2.628(3) |
| η (%) | 2.96 | 2.04 | 1.26 | 2.90 | 3.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julien, C.; Mauger, A.; Zaghib, K.; Groult, H. Optimization of Layered Cathode Materials for Lithium-Ion Batteries. Materials 2016, 9, 595. https://doi.org/10.3390/ma9070595
Julien C, Mauger A, Zaghib K, Groult H. Optimization of Layered Cathode Materials for Lithium-Ion Batteries. Materials. 2016; 9(7):595. https://doi.org/10.3390/ma9070595
Chicago/Turabian StyleJulien, Christian, Alain Mauger, Karim Zaghib, and Henri Groult. 2016. "Optimization of Layered Cathode Materials for Lithium-Ion Batteries" Materials 9, no. 7: 595. https://doi.org/10.3390/ma9070595
APA StyleJulien, C., Mauger, A., Zaghib, K., & Groult, H. (2016). Optimization of Layered Cathode Materials for Lithium-Ion Batteries. Materials, 9(7), 595. https://doi.org/10.3390/ma9070595