Electrospun Scaffolds for Corneal Tissue Engineering: A Review
Abstract
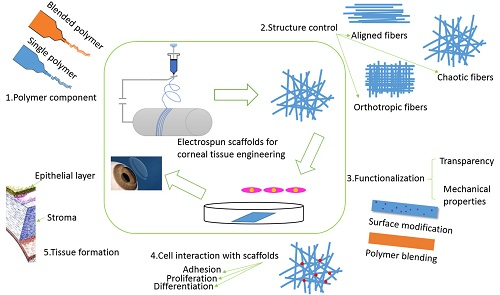
:1. Introduction
2. Materials of Electrospun Scaffolds
2.1. Single Electrospun Scaffolds for Corneal Tissue Engineering
2.2. Blended Electrospun Scaffolds for Corneal Tissue Engineering
3. Methods of Fabrication of Electrospun Scaffolds
3.1. Electrospun Scaffolds with Isotropic or Anisotropic Structure
3.1.1. Electrospun Scaffolds with Randomly Oriented Fibers
3.1.2. Electrospun Scaffolds with Aligned Fibers
3.2. Functionalization of Electrospun Scaffolds
3.2.1. Electrospun Scaffolds with Improved Transparency
3.2.2. Electrospun Scaffolds with Improved Mechanical Properties
4. Applications of Electrospun Scaffolds for Corneal Tissue Regeneration
4.1. Cell Survival and Differentiation of Corneal Cells
4.2. Maintenance of Corneal Cell Phenotype
4.3. Corneal Tissue Formation
5. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilson, S.L.; Wimpenny, I.; Ahearne, M.; Rauz, S.; El Haj, A.J.; Yang, Y. Chemical and Topographical Effects on Cell Differentiation and Matrix Elasticity in a Corneal Stromal Layer Model. Adv. Funct. Mater. 2012, 22, 3641–3649. [Google Scholar] [CrossRef]
- McLaughlin, C.R.; Tsai, R.J.F.; Latorre, M.A.; Griffith, M. Bioengineered corneas for transplantation and in vitro toxicology. Front. Biosci. 2009, 14, 3326–3337. [Google Scholar] [CrossRef]
- Ruberti, J.W.; Zieske, J.D. Prelude to corneal tissue engineering—Gaining control of collagen organization. Prog. Retin. Eye Res. 2008, 27, 549–577. [Google Scholar] [CrossRef] [PubMed]
- Ambati, B.K.; Nozaki, M.; Singh, N.; Takeda, A.; Jani, P.D.; Suthar, T.; Albuquerque, R.J.C.; Richter, E.; Sakurai, E.; Newcomb, M.T.; et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature 2006, 443, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Ortega, I.; Sefat, F.; Deshpande, P.; Paterson, T.; Ramachandran, C.; Ryan, A.J.; MacNeil, S.; Claeyssens, F. Combination of Microstereolithography and Electrospinning to Produce Membranes Equipped with Niches for Corneal Regeneration. J. Vis. Exp. 2014. [Google Scholar] [CrossRef] [PubMed]
- Quantock, A.J.; Young, R.D. Development of the Corneal Stroma, and the Collagen-Proteoglycan Associations That Help Define Its Structure and Function. Dev. Dyn. 2008, 237, 2607–2621. [Google Scholar] [CrossRef] [PubMed]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar] [PubMed]
- Wu, J.; Du, Y.; Watkins, S.C.; Funderburgh, J.L.; Wagner, W.R. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials 2012, 33, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.C.; Patel, S.V.; Hodge, D.O.; Leavitt, J.A. Corneal Sensitivity, Blink Rate, and Corneal Nerve Density in Progressive Supranuclear Palsy and Parkinson Disease. Cornea 2013, 32, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lu, Y.; Wu, T.; Zhang, M.; Zhang, Y.; Jin, Y. Construction of tissue-engineered cornea composed of amniotic epithelial cells and acellular porcine cornea for treating corneal alkali burn. Biomaterials 2013, 34, 6748–6759. [Google Scholar] [CrossRef] [PubMed]
- Couture, C.; Zaniolo, K.; Carrier, P.; Lake, J.; Patenaude, J.; Germain, L.; Guerin, S.L. The tissue-engineered human cornea as a model to study expression of matrix metalloproteinases during corneal wound healing. Biomaterials 2016, 78, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Fagerholm, P.; Lagali, N.S.; Ong, J.A.; Merrett, K.; Jackson, W.B.; Polarek, J.W.; Suuronen, E.J.; Liu, Y.; Brunette, I.; Griffith, M. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials 2014, 35, 2420–2427. [Google Scholar] [CrossRef] [PubMed]
- Guoguang, N.; Jin-San, C.; Zhan, W.; Skardal, A.; Giegengack, M.; Soker, S. Heparin-modified gelatin scaffolds for human corneal endothelial cell transplantation. Biomaterials 2014, 35, 4005–4014. [Google Scholar]
- Sharma, S.; Mohanty, S.; Gupta, D.; Jassal, M.; Agrawal, A.K.; Tandon, R. Cellular response of limbal epithelial cells on electrospun poly-epsilon-caprolactone nanofibrous scaffolds for ocular surface bioengineering: A preliminary in vitro study. Mol. Vis. 2011, 17, 2898–2910. [Google Scholar] [PubMed]
- Schwab, I.R.; Reyes, M.; Isseroff, R.R. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea 2000, 19, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, S.S.; Ng, S.L.; Chua, K.H.; Hayati, A.R.; Tan, A.E.; Tan, G.C. Value of human amniotic epithelial cells in tissue engineering for cornea. Hum. Cell 2010, 23, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Shirao, Y.; Yoshita, T.; Yagami, K.; Segawa, Y.; Kawasaki, K.; Shouzu, M.; Tseng, S.C.G. Temporary amniotic membrane patching for acute chemical burns. Eye 2003, 17, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kheirkhah, A.; Johnson, D.A.; Paranjpe, D.R.; Raju, V.K.; Casas, V.; Tseng, S.C.G. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch. Ophthalmol. Chic 2008, 126, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008, 15, 88–99. [Google Scholar]
- Lai, J.-Y.; Li, Y.-T.; Cho, C.-H.; Yu, T.-C. Nanoscale modification of porous gelatin scaffolds with chondroitin sulfate for corneal stromal tissue engineering. Int. J. Nanomed. 2012, 7, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef] [PubMed]
- Uzunalli, G.; Soran, Z.; Erkal, T.S.; Dagdas, Y.S.; Dinc, E.; Hondur, A.M.; Bilgihan, K.; Aydin, B.; Guler, M.O.; Tekinay, A.B. Bioactive self-assembled peptide nanofibers for corneal stroma regeneration. Acta Biomater. 2014, 10, 1156–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cejkova, J.; Trosan, P.; Cejka, C.; Lencova, A.; Zajicova, A.; Javorkova, E.; Kubinova, S.; Sykova, E.; Holan, V. Suppression of alkali-induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp. Eye Res. 2013, 116, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.A.; Gemeinhart, R.A.; Yue, B.Y.J.T.; Djalilian, A.R. Decellularized Human Cornea for Reconstructing the Corneal Epithelium and Anterior Stroma. Tissue Eng. Part C Methods 2012, 18, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Funamoto, S.; Sasaki, S.; Honda, T.; Hattori, S.; Nam, K.; Kimura, T.; Mochizuki, M.; Fujisato, T.; Kobayashi, H.; et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials 2010, 31, 3941–3948. [Google Scholar] [CrossRef] [PubMed]
- Bayyoud, T.; Thaler, S.; Hofmann, J.; Maurus, C.; Spitzer, M.S.; Bartz-Schmidt, K.-U.; Szurman, P.; Yoeruek, E. Decellularized Bovine Corneal Posterior Lamellae as Carrier Matrix for Cultivated Human Corneal Endothelial Cells. Curr. Eye Res. 2012, 37, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ortega, I.; Ryan, A.J.; Deshpande, P.; MacNeil, S.; Claeyssens, F. Combined microfabrication and electrospinning to produce 3-D architectures for corneal repair. Acta Biomater. 2013, 9, 5511–5520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, X.; Lu, T.J.; Xu, F. Recent Advances in Electrospun Nanofibrous Scaffolds for Cardiac Tissue Engineering. Adv. Funct. Mater. 2015, 25, 5726–5738. [Google Scholar] [CrossRef]
- Sahay, R.; Kumar, P.S.; Sridhar, R.; Sundaramurthy, J.; Venugopal, J.; Mhaisalkar, S.G.; Ramakrishna, S. Electrospun composite nanofibers and their multifaceted applications. J. Mater. Chem. 2012, 22, 12953–12971. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electro spinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Mihara, H.; Serizawa, T. Peptides as New Smart Bionanomaterials: Molecular-Recognition and Self-Assembly Capabilities. Chem. Rec. 2013, 13, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Arnoult, O.; Smith, M.; Wnek, G.E. Electrospinning of in situ crosslinked collagen nanofibers. J. Mater. Chem. 2012, 22, 19412–19417. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Cipriani, E.; Gnavi, S.; Chiono, V.; Mattu, C.; Gentile, P.; Perroteau, I.; Zanetti, M.; Ciardelli, G. Cross linked gelatin nanofibres: Preparation, characterisation and in vitro studies using glial-like cells. Mater. Sci. Eng. C 2013, 33, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Gnavi, S.; Fornasari, B.E.; Tonda-Turo, C.; Laurano, R.; Zanetti, M.; Ciardelli, G.; Geuna, S. The Effect of Electrospun Gelatin Fibers Alignment on Schwann Cell and Axon Behavior and Organization in the Perspective of Artificial Nerve Design. Int. J. Mol. Sci. 2015, 16, 12925–12942. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, X.; Zhou, S.; Weng, J. Degradation patterns and surface wettability of electrospun fibrous mats. Polym. Degrad. Stab. 2008, 93, 731–738. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Zussman, E.; Theron, A.; Yarin, A.L. Formation of nanofiber crossbars in electrospinning. Appl. Phys. Lett. 2003, 82, 973–975. [Google Scholar] [CrossRef]
- Pokorny, P.; Kostakova, E.; Sanetrnik, F.; Mikes, P.; Chvojka, J.; Kalous, T.; Bilek, M.; Pejchar, K.; Valtera, J.; Lukas, D. Effective AC needleless and collectorless electrospinning for yarn production. Phys. Chem. Chem. Phys. 2014, 16, 26816–26822. [Google Scholar] [CrossRef] [PubMed]
- Jun-Seo, P. Electrospinning and its applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 043002. [Google Scholar] [CrossRef]
- Bara, A.; Marinescu, V.; Chitanu, E.; Banciu, C.; Clicinschi, F. Influence of process parameters on the morphology of polyacrylonitrile electrospun fibers. Ind. Textila 2015, 66, 232–239. [Google Scholar]
- Wu, J.; Wang, N.; Zhao, Y.; Jiang, L. Electrospinning of multilevel structured functional micro-/nanofibers and their applications. J. Mater. Chem. A 2013, 1, 7290–7305. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Biazar, E.; Heidari-Keshel, S. Cellular Response of Limbal Stem Cells on PHBV/Gelatin Nanofibrous Scaffold for Ocular Epithelial Regeneration. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 879–887. [Google Scholar] [CrossRef]
- Ye, J.; Shi, X.; Chen, X.; Xie, J.; Wang, C.; Yao, K.; Gao, C.; Gou, Z. Chitosan-modified, collagen-based biomimetic nanofibrous membranes as selective cell adhering wound dressings in the treatment of chemically burned corneas. J. Mater. Chem. B 2014, 2, 4226–4236. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, D.; Mohanty, S.; Jassal, M.; Agrawal, A.K.; Tandon, R. Surface-Modified Electrospun Poly(epsilon-Caprolactone) Scaffold With Improved Optical Transparency and Bioactivity for Damaged Ocular Surface Reconstruction. Invest. Ophthalmol. Vis. Sci. 2014, 55, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Azari, P.; Luan, N.S.; Gan, S.N.; Yahya, R.; Wong, C.S.; Chua, K.H.; Pingguan-Murphy, B. Electrospun Biopolyesters as Drug Screening Platforms for Corneal Keratocytes. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 785–791. [Google Scholar] [CrossRef]
- Bakhshandeh, H.; Soleimani, M.; Hosseini, S.S.; Hashemi, H.; Shabani, I.; Shafiee, A.; Nejad, A.H.B.; Erfan, M.; Dinarvand, R.; Atyabi, F. Poly (epsilon-caprolactone) nanofibrous ring surrounding a polyvinyl alcohol hydrogel for the development of a biocompatible two-part artificial cornea. Int. J. nanomed. 2011, 6, 1509–1515. [Google Scholar]
- Deshpande, P.; McKean, R.; Blackwood, K.A.; Senior, R.A.; Ogunbanjo, A.; Ryan, A.J.; MacNeil, S. Using poly(lactide-co-glycolide) electrospun scaffolds to deliver cultured epithelial cells to the cornea. Regen. Med. 2010, 5, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Acun, A.; Hasirci, V. Construction of a collagen-based, split-thickness cornea substitute. J. Biomat. Sci. Polym. E 2014, 25, 1110–1132. [Google Scholar] [CrossRef] [PubMed]
- Phu, D.; Wray, L.S.; Warren, R.V.; Haskell, R.C.; Orwin, E.J. Effect of Substrate Composition and Alignment on Corneal Cell Phenotype. Tissue Eng. Part A 2011, 17, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Wray, L.S.; Orwin, E.J. Recreating the Microenvironment of the Native Cornea for Tissue Engineering Applications. Tissue Eng. Part A 2009, 15, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Biazar, E.; Baradaran-Rafii, A.; Heidari-keshel, S.; Tavakolifard, S. Oriented nanofibrous silk as a natural scaffold for ocular epithelial regeneration. J. Biomat. Sci. Polym. E 2015, 26, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Tonsomboon, K.; Oyen, M.L. Composite electrospun gelatin fiber-alginate gel scaffolds for mechanically robust tissue engineered cornea. J. Mech. Behav. Biomed. 2013, 21, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Bahners, T.; Gutmann, J.S.; Gao, S.L.; Maeder, E.; Fuchsluger, T.A. Characterization of structural, mechanical and nano-mechanical properties of electrospun PGS/PCL fibers. RSC Adv. 2014, 4, 16951–16957. [Google Scholar] [CrossRef]
- Yan, J.; Qiang, L.; Gao, Y.; Cui, X.; Zhou, H.; Zhong, S.; Wang, Q.; Wang, H. Effect of fiber alignment in electrospun scaffolds on keratocytes and corneal epithelial cells behavior. J. Biomed. Mater. Res. A 2012, 100A, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wen, J.; Yan, J.; Kao, Y.; Ni, Z.; Cui, X.; Wang, H. In situ growth induction of the corneal stroma cells using uniaxially aligned composite fibrous scaffolds. RSC Adv. 2015, 5, 12123–12130. [Google Scholar] [CrossRef]
- Chen, J.; Yan, C.; Zhu, M.; Yao, Q.; Shao, C.; Lu, W.; Wang, J.; Mo, X.; Gu, P.; Fu, Y.; et al. Electrospun nanofibrous SF/P(LLA-CL) membrane: A potential substratum for endothelial keratoplasty. Int. J. Nanomed. 2015, 10, 3337–3350. [Google Scholar]
- Kharaziha, M.; Nikkhah, M.; Shin, S.-R.; Annabi, N.; Masoumi, N.; Gaharwar, A.K.; Camci-Unal, G.; Khademhosseini, A. PGS: Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials 2013, 34, 6355–6366. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Venugopal, J.R.; Mueller, M.; Sundarrajan, S.; Mukherjee, S.; Pliska, D.; Wintermantel, E.; Ramakrishna, S. Buckled structures and 5-azacytidine enhance cardiogenic differentiation of adipose-derived stem cells. Nanomedicine (Lond.) 2013, 8, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Prabhakaran, M.P.; Kai, D.; Ramakrishna, S. Human cardiomyocyte interaction with electrospun fibrinogen/gelatin nanofibers for myocardial regeneration. J. Biomat. Sci. Polym. E 2013, 24, 1660–1675. [Google Scholar] [CrossRef] [PubMed]
- Kenar, H.; Kose, G.T.; Hasirci, V. Design of a 3D aligned myocardial tissue construct from biodegradable polyesters. J. Mater. Sci. Mater. Med. 2010, 21, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Walthall, J.M.; Venkataraman, R.; Crowder, S.W.; Jung, D.K.; Yu, S.S.; Feaster, T.K.; Wang, X.; Giorgio, T.D.; Hong, C.C.; et al. Combinatorial Polymer Electrospun Matrices Promote Physiologically-Relevant Cardiomyogenic Stem Cell Differentiation. PLoS ONE 2011, 6, e28935. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.; D’Aquino, R.; Angelis, M.G.C.-D.; De Francesco, F.; Giordano, A.; Laino, G.; Piattelli, A.; Traini, T.; De Rosa, A.; Papaccio, G. Scaffold’s surface geometry significantly affects human stem cell bone tissue engineering. J. Cell. Physiol. 2008, 214, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Venugopal, J.; Prabhakaran, M.P.; Dev, V.R.G.; Low, S.; Choon, A.T.; Ramakrishna, S. Aligned and random nanofibrous substrate for the in vitro culture of Schwann cells for neural tissue engineering. Acta Biomater. 2009, 5, 2560–2569. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Teo, W.E.; Zhu, X.; Beuerman, R.W.; Ramakrishna, S.; Yung, L.Y.L. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J. Biomed. Mater. Res. A 2006, 79A, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Kwon, O.H.; Meng, W.; Kang, I.K. Nanofabrication of microbial polyester by electrospinning promotes cell attachment. Macromol. Res. 2004, 12, 374–378. [Google Scholar] [CrossRef]
- Lee, C.H.; Shin, H.J.; Cho, I.H.; Kang, Y.M.; Kim, I.A.; Park, K.D.; Shin, J.W. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials 2005, 26, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.M.; Weber, H.J. Electrospinning P(LLA-CL) nanofiber: A tubular scaffold fabrication with circumferential alignment. Macromol. Symp. 2004, 217, 413–416. [Google Scholar] [CrossRef]
- Katta, P.; Alessandro, M.; Ramsier, R.D.; Chase, G.G. Continuous electrospinning of aligned polymer nanofibers onto a wire drum collector. Nano Lett. 2004, 4, 2215–2218. [Google Scholar] [CrossRef]
- Kameoka, J.; Orth, R.; Yang, Y.N.; Czaplewski, D.; Mathers, R.; Coates, G.W.; Craighead, H.G. A scanning tip electrospinning source for deposition of oriented nanofibres. Nanotechnology 2003, 14, 1124–1129. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.L.; Xia, Y.N. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Kakade, M.V.; Givens, S.; Gardner, K.; Lee, K.H.; Chase, D.B.; Rabolt, J.F. Electric field induced orientation of polymer chains in macroscopically aligned electrospun polymer nanofibers. J. Am. Chem. Soc. 2007, 129, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Li, L.; Hu, L.; Cui, X. Continuous aligned polymer fibers produced by a modified electrospinning method. Polymer 2006, 47, 4901–4904. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Mi, S.; Kong, B.; Wu, Z.; Sun, W.; Xu, Y.; Su, X. A novel electrospinning setup for the fabrication of thickness-controllable 3D scaffolds with an ordered nanofibrous structure. Mater. Lett. 2015, 160, 343–346. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, S.J.; Christ, G.J.; Atala, A.; Yoo, J.J. The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 2008, 29, 2899–2906. [Google Scholar] [CrossRef] [PubMed]
- Ceria, A.; Hauser, P.J. Atmospheric plasma treatment to improve durability of a water and oil repellent finishing for acrylic fabrics. Surf. Coat. Technol. 2010, 204, 1535–1541. [Google Scholar] [CrossRef]
- Jester, J.V.; Moller-Pedersen, T.; Huang, J.Y.; Sax, C.M.; Kays, W.T.; Cavangh, H.D.; Petroll, W.M.; Piatigorsky, J. The cellular basis of corneal transparency: Evidence for ‘corneal crystallins’. J. Cell Sci. 1999, 112, 613–622. [Google Scholar] [PubMed]
- Jester, J.V. Corneal crystallins and the development of cellular transparency. Semin. Cell Dev. Biol. 2008, 19, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Budge, A.; Fisher, S.; Huang, J.Y. Corneal keratocytes: Phenotypic and species differences in abundant protein expression and in vitro light-scattering. Invest. Ophthalmol. Vis. Sci. 2005, 46, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Seitz, V.; Venugopal, J.R.; Sridhar, R.; Sundarrajan, S.; Mukherjee, S.; Wintermantel, E.; Ramakrishna, S. Mimicking Native Extracellular Matrix with Phytic Acid-Crosslinked Protein Nanofibers for Cardiac Tissue Engineering. Macromol. Biosci. 2013, 13, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Baradaran-Rafii, A.; Biazar, E.; Heidari-Keshel, S. Cellular Response of Limbal Stem Cells on Polycaprolactone Nanofibrous Scaffolds for Ocular Epithelial Regeneration. Curr. Eye Res. 2016, 41, 326–333. [Google Scholar] [CrossRef] [PubMed]








| Polymer | Solvent | Concentration | Fiber Diameter | Cell Type | Advantages | Advanced Properties | Ref. |
|---|---|---|---|---|---|---|---|
| Single polymer | |||||||
| PCL | TFE | 10% w/v | 90–174 nm | Limbal epithelial cell | Biocompatible, able to retain a normal corneal phenotype, promote corneal epithelium formation | [14] | |
| TFE | 10% w/v | 108–172 nm | Human corneal epithelial cell | Bioactive and biocompatible, improved cell attachment | Functionalized by He/O2 plasma | [44] | |
| Chloroform/DMF | 10% w/v | ≈310 nm | Rabbit keratocytes | Promote cell attachment and proliferation | [45] | ||
| Chloroform/DMF | 5% w/v | 400–800 nm | Rabbit limbal stem cells | Improve mechanical properties, cell attachment and proliferation | Functionalized by plasma | [46] | |
| PLDLA | Chloroform/DMF | 2% w/v | Human corneal stromal cells | Biocompatible, promote reverting corneal fibroblasts to a keratocyte phenotype | Orthogonal multilayers, aligned fibers for each layer | [1] | |
| PLGA | Dichloromethane | 25% w/v | 40–130 nm | Rabbit limbal fibroblasts and rabbit limbal epithelial cells | FDA-approved and artificial bionic limbus | Combined with microstereolithography | [27] |
| Dichloromethane | 25% w/v | Rabbit limbal epithelial cells | Biocompatible, promote multilayer formation of cells | [47] | |||
| PHBV | Chloroform/DMF | 10% w/v | ≈1350 nm | Rabbit keratocytes | Promote cell attachment and proliferation | [45] | |
| PEUU | HFIP | 5% w/v | 100–220 nm | Human corneal stromal stem cells | Promote the differentiation of stem cells to keratocytes and production of collagen matrix | Aligned fibers | [8] |
| Collagen | HFIP/DMF | 9% w/v | Retinal pigment epithelium cells and human corneal keratocytes | Suitable for cell attachment and growth and more ECM deposition | High transparency | [48] | |
| Acetic acid | 4%–7.5% w/v | 50–451 nm | Rabbit corneal fibroblasts | Biocompatible, reduced myofibroblast phenotype expression on aligned scaffold | Aligned fibers | [49] | |
| Acetic acid | 4%–7.5% w/v | 160–240 nm | Rabbit corneal fibroblasts | Suitable for cell attachment and growth | Aligned fibers | [50] | |
| Silk | TFE | 2.5% w/v | Human limbal stem cells | Biocompatible, promote corneal epithelium formation | Aligned fibers | [51] | |
| Gelatin | Glacial acetic acid/ethylacetate/distilled water | 10% w/v | 60–148 nm | Improved mechanical properties | Aligned fiber-alginate gel and improved transparency | [52] | |
| Blended polymer | |||||||
| PHBV/Gelatin | TFE | 50% w/v | ≈100 nm | Limbal stem cell | Biocompatible, promote cell attachment and proliferation and corneal epithelium formation | Improved transparency | [42] |
| PGS/PCL | Chloroform/ethanol | 13% w/v | 300–550 nm | Human corneal epithelial cell | Increased moduli | Aligned fibers | [53] |
| Collagen/HA/PEO | Acetic acid | 10% w/v | 51.3–106.9 nm | Epithelial cells, fibroblasts | Excellent biocompatibility and mechanical properties, promote cell attachment and corneal epithelium regeneration | Chitosan surface modified and improved transparency | [43] |
| Gelatin/PLLA | HFIP/DMF | 5% w/v | 800–1000 nm | Corneal epithelial cells and keratocytes | Biocompatible, improved mechanical properties | Aligned fibers | [54] |
| HFIP/DMF | 10% w/v | 750–1000 nm | Improve the regeneration of corneal stroma | Aligned fibers and improved transparency | [55] | ||
| SF/P(LLA-CL) | HFIP | 8% w/v | 123–649 nm | Human corneal endothelial cells | Promote mechanical properties, cell attachment and proliferation | Improved transparency | [56] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, B.; Mi, S. Electrospun Scaffolds for Corneal Tissue Engineering: A Review. Materials 2016, 9, 614. https://doi.org/10.3390/ma9080614
Kong B, Mi S. Electrospun Scaffolds for Corneal Tissue Engineering: A Review. Materials. 2016; 9(8):614. https://doi.org/10.3390/ma9080614
Chicago/Turabian StyleKong, Bin, and Shengli Mi. 2016. "Electrospun Scaffolds for Corneal Tissue Engineering: A Review" Materials 9, no. 8: 614. https://doi.org/10.3390/ma9080614
APA StyleKong, B., & Mi, S. (2016). Electrospun Scaffolds for Corneal Tissue Engineering: A Review. Materials, 9(8), 614. https://doi.org/10.3390/ma9080614