Improvement in the Tensile Bond Strength between 3Y-TZP Ceramic and Enamel by Surface Treatments
Abstract
:1. Introduction
2. Materials and Methods
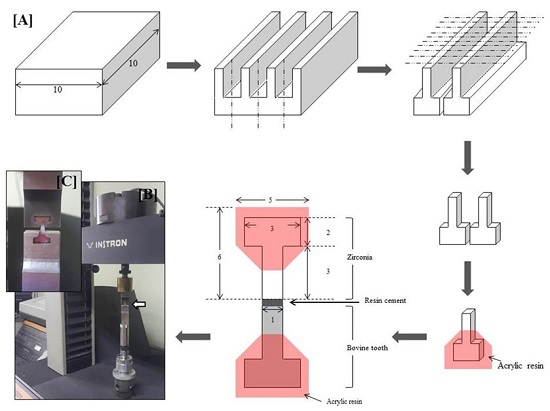
2.1. Preparation of Specimens and Surface Treatments
2.2. Surface Roughness, Wettability, and Morphology
2.3. Tensile Bond Strength (TBS) Test and Failure Modes
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- Starting with the lowest, the order of TBS values for different zirconia surface treatments was the P group, the S group, the SS group, and the SP group.
- An increase in the TBS value of the treated surface was accompanied by a concomitantly higher rate of cohesive failure at the bonded surface between the treated zirconia and enamel.
- Compared to the P group, the roughness of zirconia increased significantly after surface treatments (p < 0.05); moreover, the contact angle decreased significantly (p < 0.05) while the wettability increased, which indicated a strong relationship between surface roughness and wettability.
- The surface treatment method of applying MDP-containing silane primer after 110 µm Al2O3 sandblasting (SP group) is clinically convenient and effective at providing considerable improvements in the chemical bond strength between the zirconia and enamel.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.M.; Sakaguchi, R.L. Craig’s Restorative Dental Materials, 15th ed.; Elsevier Mosby: Philadelphia, PA, USA, 2012; pp. 455–462. [Google Scholar]
- Yenisey, M.; Dede, D.Ö.; Rona, N. Effect of surface treatments on the bond strength between resin cement and differently sintered zirconium-oxide ceramics. J. Prosthodont. Res. 2016, 60, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.; Özcan, M.; Bottino, M.A.; Valandro, L.F. Microtensile bond strength of a resin cement to glass infiltrated zirconia-reinforced ceramic: The effect of surface conditioning. Dent. Mater. 2006, 22, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Papia, E.; Larsson, C.; Toit, M.; Steyern, P.V. Bonding between oxide ceramics and adhesive cement systems: A systematic review. J. Biomed. Mater. Res. Part B 2014, 102, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.A.; Ahn, J.S.; Park, Y.J.; Jun, S.H.; Lee, I.B.; Cho, B.H.; Son, H.H.; Seo, D.G. The effect of sandblasting and different primers on shear bond strength between yttria-tetragonal zirconia polycrystal ceramic and a self-adhesive resin cement. Oper. Dent. 2015, 40, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.; Hooshmand, T.; Heidari, S.; Khoshro, K. Effect of sandblasting, silica coating, and laser treatment on the microtensile bond strength of a dental zirconia ceramic to resin cements. Lasers Med. Sci. 2016, 31, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Yi, Y.A.; Lee, Y.; Seo, D.G. Shear bond strength of MDP-containing self-adhesive resin cement and Y-TZP ceramics: Effect of phosphate monomer-containing primers. Biomed. Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pilo, R.; Kaitsas, V.; Zinelis, S.; Eliades, G. Interaction of zirconia primers with yttria-stabilized zirconia surfaces. Dent. Mater. 2016, 32, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Fukegawa, D.; Hayakawa, S.; Yoshida, Y.; Suzuki, K.; Osaka, A.; Meerbeek, V.B. Chemical interaction of phosphoric acid ester with hydroxyapatite. J. Dent. Res. 2006, 85, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Ebenezar, A.; Anand, N.; Rajkumar, K.; Mahalaxmi, S.; Srinivasan, N. Microshear bond strength evaluation of surface pretreated zirconia ceramics bonded to dentin. Eur. J. Dent. 2015, 9, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Inokoshi, M.; Munck, D.J.; Minakuchi, S.; Meerbeek, V.B. Meta-analysis of bonding effectiveness to zirconia ceramics. J. Dent. Res. 2014, 93, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Akin, H.; Tugut, F.; Akin, G.E.; Guney, U.; Mutaf, B. Effect of Er: YAG laser application on the shear bond strength and microleakage between resin cements and Y-TZP ceramics. Lasers Med. Sci. 2012, 27, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Park, D.R.; Kim, J.M.; Jeoung, S.H.; Bae, J.M.; Oh, S.H. The effect of selective infiltration etching technique on the shear bond strength between zirconia and veneering ceramic. Korean Soc. Dent. Mater. 2014, 41, 113–120. [Google Scholar] [CrossRef]
- Pashley, D.H.; Carvalho, R.M.; Sano, H.; Nakajima, M.; Yoshiyama, M.; Shono, Y.; Fernandes, C.A.; Tay, F. The microtensile bond test: A review. J. Adhes. Dent. 1999, 1, 299–309. [Google Scholar] [PubMed]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carcal, P. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef]
- ISO (International Organization for Standardization). ISO/TS 11405: 2003, Dental Materials-Testing of Adhesion to Tooth Structure; ISO: Geneva, Switzerland, 2003. [Google Scholar]
- Seo, D.G. Bonding for dental ceramic. J. Korean Dent. Assoc. 2012, 50, 377–383. [Google Scholar]
- Anusavice, K.J. Phillips’ Science of Dental Materials, 11th ed.; Elsevier Mosby: Philadelphia, PA, USA, 2003. [Google Scholar]
- Bona, A.D.; Borba, M.; Benetti, P.; Cecchetti, D. Effect of surface treatments on the bond strength of a zirconia-reinforced ceramic to composite resin. Braz. Oral. Res. 2007, 21, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ishii, R.; Tsujimoto, A.; Takamizawa, T.; Tsubota, K.; Suzuki, T.; Shimamura, Y.; Miyazaki, M. Influence of surface treatment of contaminated zirconia on surface free energy and resin cement bonding. Dent. Mater. J. 2015, 34, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Zandparsa, R.; Talua, N.A.; Finkelman, M.D.; Schaus, S.E. An in vitro comparison of shear bond strength of zirconia to enamel using different surface treatments. J. Prosthodont. 2014, 23, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Vallittu, P.K. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent. Mater. 2003, 19, 725–731. [Google Scholar] [CrossRef]
- Byeon, S.M.; Nguyen, T.D.T.; Lee, M.H.; Bae, T.S. Effect of surface treatment conditions of zirconia on the shear bond strength of orthodontic metal bracket. Korean J. Dent. Mater. 2015, 42, 317–326. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Matinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2012, 28, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Sadan, A.; Martin, J.; Lang, B. In vitro evaluation of shear bond strengths of resin to densely-sintered high-purity zirconium-oxide ceramic after long-term storage and thermal cycling. J. Prosthet. Dent. 2004, 91, 356–362. [Google Scholar] [CrossRef] [PubMed]





| Groups (n = 15) | Surface Treatments |
|---|---|
| P | Polishing |
| S | 110 µm A12O3 Sandblasting |
| SS | 110 µm A12O3 Sandblasting + Selective Infiltration Etching (SIE) (i) Glass coating; (ii) Heating at 900 °C for 1 min; (iii) Cooling at 750 °C for 1 min; (iv) Re-heating at 900 °C for 20 min; (v) Cooling at room temperature; (vi) 5% HF etching for 30 min in an ultrasonic bath; (vii) Washing with distilled water for 5 min in an ultrasonic bath |
| SP | 110 µm A12O3 Sandblasting + MDP-containing silane primer |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byeon, S.-M.; Jang, Y.-S.; Lee, M.-H.; Bae, T.-S. Improvement in the Tensile Bond Strength between 3Y-TZP Ceramic and Enamel by Surface Treatments. Materials 2016, 9, 702. https://doi.org/10.3390/ma9080702
Byeon S-M, Jang Y-S, Lee M-H, Bae T-S. Improvement in the Tensile Bond Strength between 3Y-TZP Ceramic and Enamel by Surface Treatments. Materials. 2016; 9(8):702. https://doi.org/10.3390/ma9080702
Chicago/Turabian StyleByeon, Seon-Mi, Yong-Seok Jang, Min-Ho Lee, and Tae-Sung Bae. 2016. "Improvement in the Tensile Bond Strength between 3Y-TZP Ceramic and Enamel by Surface Treatments" Materials 9, no. 8: 702. https://doi.org/10.3390/ma9080702
APA StyleByeon, S. -M., Jang, Y. -S., Lee, M. -H., & Bae, T. -S. (2016). Improvement in the Tensile Bond Strength between 3Y-TZP Ceramic and Enamel by Surface Treatments. Materials, 9(8), 702. https://doi.org/10.3390/ma9080702