Effects of Increasing pH on Nitrous Oxide and Dinitrogen Emissions from Denitrification in Sterilized and Unsterilized Forest Soils
Abstract
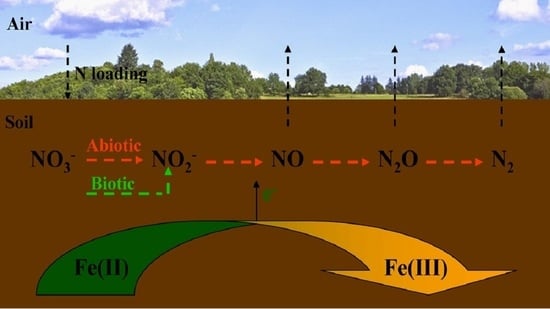
:1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sample Collection
2.2. Soil Pretreatment
2.3. Experimental Set-Up
2.4. Sterilization
2.5. Nitrogenous Gas and Aqueous Phase Analysis
2.6. Statistical Analyses
3. Results
3.1. Dynamic Changes in Nitrate Concentration
3.2. Dynamic Changes in Nitrite Concentration
3.3. Dynamic Changes in N2O and N2
3.4. Dynamic Changes in (N2O+N2) and N2O-N/(N2O+N2)-N Product Ratio
4. Discussion
4.1. The Potential Importance of Abiotic Denitrification to N2O Emission
4.2. The Effect of pH on Denitrification Rate
4.3. The Effect of pH on N2O Emission and Gaseous Product Stoichiometry
4.4. Differences in the Responses of Abiotic and Biotic Denitrification to Soil pH
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, L.; Allen, S.; Bindoff, N.L.; Breon, F.-M. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 659–740. [Google Scholar]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Chang. 2012, 2, 410–416. [Google Scholar] [CrossRef]
- Schlesinger, W.H. On the fate of anthropogenic nitrogen. Proc. Natl. Acad. Sci. USA 2009, 106, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Kanter, D.; Mauzerall, D.L.; Ravishankarac, A.R.; Danile, J.S.; Portmann, R.W.; Grabiel, P.M.; Moomaw, W.R.; Galloway, J.N. A post-Kyoto partner: Considering the stratospheric ozone regime as a tool to manage nitrous oxide. Proc. Natl. Acad. Sci. USA 2013, 110, 4451–4457. [Google Scholar] [CrossRef] [PubMed]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef]
- Seitzinger, S.P.; Kroeze, C.; Styles, R.V. Global distribution of N2O emissions from aquatic systems: Natural emissions and anthropogenic effects. Chemosphere Glob. Chang. Sci. 2000, 2, 267–279. [Google Scholar] [CrossRef]
- Conrad, R. Soil microbial processes and the cycling of atmospheric trace gases. Philos. Trans. R. Soc. Lond. 1995, 351, 219–230. [Google Scholar]
- Wang, M.L.; Hu, R.G.; Ruser, R.; Schmidt, C.; Kappler, A. Role of chemodenitrification for N2O emissions from nitrate reduction in rice paddy soils. ACS Earth Space Chem. 2020, 4, 122–132. [Google Scholar] [CrossRef]
- Heil, J.; Vereecken, H.; Brüggemann, N. A review of chemical reactions of nitrification intermediates and their role in nitrogen cycling and nitrogen trace gas formation in soil. Eur. J. Soil Sci. 2016, 67, 23–39. [Google Scholar] [CrossRef]
- Matocha, C.J.; Dhakal, P.; Pyzola, S.M. The role of abiotic and coupled biotic/abiotic mineral controlled redox processes in nitrate reduction. Adv. Agron. 2012, 115, 181–214. [Google Scholar]
- Braker, G.; Conrad, R. Diversity, structure, and size of N2O-producing microbial communities in soils-what matters for their functing? Adv. Appl. Microbiol. 2011, 75, 33–57. [Google Scholar]
- Mosier, A.R. Soil processes and global change. Biol. Fertil. Soils 1998, 27, 221–229. [Google Scholar] [CrossRef]
- Regaert, D.; Aubinet, M.; Moureaux, C. Mitigating N2O emissions from agriculture: A review of the current knowledge on soil system modeling, environmental factors and management practices influencing emissions. J. Soil Sci. Environ. Manag. 2015, 6, 178–186. [Google Scholar]
- Zumft, W. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar] [PubMed]
- Shoun, H.; Fushinobu, S.; Jiang, L.; Kim, S.W.; Wakagi, T. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Philos. Trans. R. Soc. B 2012, 367, 1186–1194. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Cha, D.K. Effect of low temperature on abiotic and biotic nitrate reduction by zero-valent iron. Sci. Total Environ. 2021, 754, 142410. [Google Scholar] [CrossRef]
- Robinson, T.C.; Latta, D.E.; Notini, L.; Schilling, K.E.; Scherer, M.M. Abiotic reduction of nitrite by Fe(II): A comparison of rates and N2O production. Environ. Sci. Process. Impacts 2021, 23, 1531. [Google Scholar] [CrossRef]
- Chen, G.J.; Zhao, W.Q.; Yang, Y.; Chen, D.D.; Wang, Y.; Li, F.B.; Zhao, Z.Y.; Cao, F.; Liu, T.X. Chemodenitrification by Fe(II) and nitrite: Effects of temperature and dual N-O isotope fractionation. Chem. Geol. 2021, 575, 120258. [Google Scholar] [CrossRef]
- Chen, D.D.; Yuan, X.; Zhao, W.Q.; Luo, X.B.; Li, F.B.; Liu, T.X. Chemodenitrification by Fe(II) and nitrite: pH effect, mineralization and kinetic modeling. Chem. Geol. 2020, 541, 119586. [Google Scholar] [CrossRef]
- Dhakal, P.; Coyne, M.S.; McNear, D.H.; Wendroth, O.O.; Vandiviere, M.M.; D’Angelo, E.M.; Matocha, C.J. Reactions of nitrite with goethite and surface Fe(II)-goethite complexes. Sci. Total Environ. 2021, 782, 146406. [Google Scholar] [CrossRef]
- Picardal, F. Abiotic and microbial interactions during anaerobic transformations of Fe(II) and NOx−. Front. Microbiol. 2012, 3, 112. [Google Scholar] [CrossRef]
- Wang, Y.J.; Cao, W.C.; Zhang, X.M.; Guo, J.H. Abiotic nitrate loss and nitrogenous trace gas emission from Chinese acidic forest soils. Environ. Sci. Pollut. Res. 2017, 24, 22679–22687. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Cao, W.C.; Zhang, X.M.; Guo, J.H. Rate of denitrification and stoichiometry of its products in fluvo-aquic Cambisols for sterilized and unsterilized incubations. Eur. J. Soil Sci. 2019, 70, 530–539. [Google Scholar] [CrossRef]
- Liu, B.B.; Mørkved, P.T.; Frostegård, Å.; Bakken, L.R. Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol. Ecol. 2010, 72, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Raut, N.; Dörsch, P.; Sitaula, B.K.; Bakken, L.R. Soil acidification by intensified crop production in South Asia results in higher N2O/(N2+N2O) product rations of denitrification. Soil Biol. Biochem. 2012, 55, 104–112. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, J.G.; Almøy, T.; Bakken, L.R. Excessive use of nitrogen in Chinese agricultural results in high N2O/(N2O+N2) product ratio of denitrification, primarily due to acidification of the soils. Glob. Chang. Biol. 2014, 20, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Van Cleemput, O. Subsoils: Chemo- and biological denitrification, N2O and N2 emissions. Nutr. Cycl. Agroecosyst. 1998, 52, 187–194. [Google Scholar] [CrossRef]
- Bergaust, L.; Mao, Y.J.; Bakken, L.R.; Frostegård, Å. Denitrification response patterns during the transition to anoxic respiration and posttranscriptional effects of suboptimal pH on nitrogen oxide reductase in Paracoccus denitrificans. Appl. Environ. Microbiol. 2010, 76, 6387–6396. [Google Scholar] [CrossRef]
- Čuhel, J.; Šimek, M. Proximal and distal control by pH of denitrification rate in a pasture soil. Agric. Ecosyst. Environ. 2011, 141, 230–233. [Google Scholar] [CrossRef]
- Philippot, L.; Andert, J.; Jones, C.M.; Bru, A.; Hallin, S. Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O emissions from soil. Glob. Chang. Biol. 2011, 17, 1497–1504. [Google Scholar] [CrossRef]
- Hansen, H.C.B.; Guldberg, S.; Erbs, M.; Koch, C.B. Kinetics of nitrate reduction by green rusts-effects of interlayer anion and Fe(II):Fe(II) ratio. Appl. Clay Sci. 2001, 18, 81–91. [Google Scholar] [CrossRef]
- Ottley, C.J.; Davison, W.; Edmunds, W.M. Chemical catalysis of nitrate reduction by iron(II). Geochim. Cosmochim. Acta 1997, 61, 1819–1828. [Google Scholar] [CrossRef]
- Choi, J.; Batchelor, B.; Won, C.; Chung, J. Nitrate reduction by green rusts modified with trace metals. Chemosphere 2012, 86, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Zhu, J.X.; Wang, Q.F.; Xu, L.; Li, M.X.; Dai, G.H.; Mulder, J.; Xi, Y.; He, N.P. Soil acidification in China’s forests due to atmospheric acid deposition from 1980 to 2050. Sci. Bull. 2022, 67, 914–917. [Google Scholar] [CrossRef]
- Zhu, J.X.; He, N.P.; Wang, Q.F.; Yuan, G.F.; Wen, D.; Yu, G.R.; Jia, Y.L. The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci. Total Environ. 2015, 511, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Tarkalson, D.D.; Payero, J.O.; Hergert, G.W.; Cassman, K.G. Acidification of soil in a dry land winter wheat-sorghum/corn-fallow rotation in the semiarid U.S. Great Plains. Plant Soil 2006, 283, 367–379. [Google Scholar] [CrossRef]
- Molstad, L.; Dörsch, P.; Bakken, L.R. Robotized incubation system for monitoring gases (O2, NO, N2O, N2) in denitrifying cultures. J. Microbiol. Methods 2007, 71, 202–211. [Google Scholar] [CrossRef]
- Hartel, P.G.; Alexander, M. Decline of cowpea rhizobia in acid soils after gamma-irradiation. Soil Biol. Biochem. 1983, 15, 489–490. [Google Scholar] [CrossRef]
- Quin, P.; Joseph, S.; Husson, O.; Donne, S.; Mitchell, D.; Munroe, P.; Phelan, D.; Cowie, A.; Van Zwieten, L. Lowering N2O emissions from soils using eucalypt biochar: The importance of redox reactions. Sci. Rep. 2015, 5, 16773. [Google Scholar] [CrossRef]
- Wei, J.; Amelung, W.; Lehndorff, E.; Schloter, M.; Vereecken, H.; Brüggemann, N. N2O and NOx emissions by reactions of nitrite with soil organic matter of a Norway spruce forest. Biogeochemistry 2017, 132, 325–342. [Google Scholar] [CrossRef]
- Parkin, T.B.; Sexstone, A.J.; Tiedje, J.M. Adaptation of denitrifying populations to low soil pH. Appl. Environ. Microbiol. 1985, 49, 1053–1056. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, 1732. [Google Scholar] [CrossRef] [PubMed]
- Nägele, W.; Conrad, R. Influence of soil pH on the nitrate-reducing microbial populations and their potential to reduce nitrate to NO and N2O. FEMS Microbiol. Ecol. 1990, 74, 49–58. [Google Scholar] [CrossRef]
- Venterea, R.T. Nitrite-driven nitrous oxide production under aerobic soil conditions: Kinetics and biochemical controls. Glob. Chang. Biol. 2007, 13, 1798–1809. [Google Scholar] [CrossRef]
- Sørensen, J.; Thorling, L. Stimulation by lepidocrocite (γ-FeOOH) of Fe(II)-dependent nitrite reduction. Geochim. Cosmochim. Acta 1991, 55, 1289–1294. [Google Scholar] [CrossRef]
- Samarkin, V.A.; Madigan, M.T.; Bowles, M.W.; Casciotti, K.L.; Priscu, J.C.; Mckay, C.P.; Joye, S.B. Abiotic nitrous oxide emission from the hypersaline Don Juan Pond in Antarctica. Nat. Geosci. 2010, 3, 341–344. [Google Scholar] [CrossRef]
- Dhakal, P.; Matocha, C.J.; Huggins, F.E.; Vandiviere, M.M. Nitrite reactivity with magnetite. Environ. Sci. Technol. 2013, 47, 6206–6213. [Google Scholar] [CrossRef]
- Philippot, L.; Hallin, S.; Schloter, M. Ecology of denitrifying prokaryotes in agricultural soil. Adv. Agron. 2007, 96, 250–305. [Google Scholar]
- Richardson, D.; Felgate, H.; Watmough, N.; Thomson, A.; Baggs, E. Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle—Could enzymic regulation hold the key? Trends Biotechnol. 2009, 27, 388–397. [Google Scholar] [CrossRef]
- Bollag, J.M.; Orcutt, M.L.; Bollag, B. Denitrification by isolated soil bacteria under various environmental conditions. Soil Sci. Soc. Am. J. 1970, 34, 875–879. [Google Scholar] [CrossRef]
- Townsend, L.R. Effect of form of N and pH on nitrate reductase activity in lowbush blueberry leaves and roots. Can. J. Plant Sci. 1970, 50, 603–605. [Google Scholar] [CrossRef]
- Šimek, M.; Cooper, J.E. The influence of soil pH on denitrification: Progress towards the understanding of this interaction over the last 50 years. Eur. J. Soil Sci. 2002, 53, 345–354. [Google Scholar] [CrossRef]
- Farquharson, R.; Baldock, J. Concepts in modeling N2O emissions from land use. Plant Soil 2008, 309, 147–167. [Google Scholar] [CrossRef]
- Etique, M.; Zegeye, A.; Grégoire, B.; Carteret, C.; Ruby, C. Nitrate reduction by mixed iron (II-III) hydroxycarbonate green rust in the presence of phosphate anions: The key parameters influencing the ammonium selectivity. Water Res. 2014, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Chorover, J.; Dail, D.B. A mechanism of abiotic immobilization of nitrate in forest ecosystems: The ferrous wheel hypothesis. Glob. Chang. Biol. 2003, 9, 228–236. [Google Scholar] [CrossRef]
- Wang, Y.J.; Liu, J.; Guo, J.H. Chemical transformation of soil nitrogen under the influence of iron: A review. J. China Agric. Univ. 2014, 19, 95–99. [Google Scholar]
- Čuhel, J.; Šimek, M.; Laughlin, R.J.; Bru, D.; Chèneby, D.; Watson, C.J.; Philippot, L. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl. Environ. Microbio. 2010, 76, 1870–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obia, A.; Cornelissen, G.; Mulder, J.; Dörsch, P. Effect of soil pH increased by biochar on NO, N2O and N2 production during denitrification in acid soils. PLoS ONE 2015, 10, e0138781. [Google Scholar] [CrossRef]
- Shaaban, M.; Peng, Q.A.; Hu, R.G.; Wu, Y.P.; Lin, S.; Zhao, J.S. Dolomite application to acidic soils: A promising option for mitigating N2O emissions. Environ. Sci. Pollut. Res. 2015, 22, 19961–19970. [Google Scholar] [CrossRef]
- Moraghan, J.T.; Buresh, R.J. Chemical reduction of nitrite and nitrous oxide by ferrous iron. Soil Sci. Soc. Am. J. 1977, 41, 47–50. [Google Scholar] [CrossRef]
- Rakshit, S.; Matocha, C.J.; Haszler, G.R. Nitrate reduction in the presence of wüstite. J. Environ. Qual. 2005, 34, 1286–1292. [Google Scholar] [CrossRef]
- Van Hecke, K.; Van Cleemput, O.; Baert, L. Chemo-denitrification of nitrate-polluted water. Environ. Pollut. 1990, 63, 261–274. [Google Scholar] [CrossRef]
- Homyak, P.M.; Kamiyama, M.; Sickman, J.O.; Schimel, J.P. Acidity and organic matter promote abiotic nitric oxide production in drying soils. Glob. Chang. Biol. 2017, 23, 1735–1747. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Original Soil pH 5.5 | After Pre-Incubation | |||
|---|---|---|---|---|---|
| Sterilized | Unsterilized | ||||
| pH 5.5 | pH 7.1 | pH 5.5 | pH 7.1 | ||
| Moisture (%) | 29.6 ± 0.1 | 29.3 ± 0.1 | 29.1 ± 0.1 | 29.3 ± 0.1 | 29.1 ± 0.1 |
| NO3− (mg N kg−1) | 19.8 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| NO2− (mg N kg−1) | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.0 ± 0.0 |
| Total Fe (mg kg−1) | 639.3 ± 29.3 | 779.8 ± 15.4 | 704.6 ± 9.4 | 811.3 ± 11.6 | 751.4 ± 15.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cao, W.; Guo, J.; Zhang, M. Effects of Increasing pH on Nitrous Oxide and Dinitrogen Emissions from Denitrification in Sterilized and Unsterilized Forest Soils. Forests 2022, 13, 1589. https://doi.org/10.3390/f13101589
Wang Y, Cao W, Guo J, Zhang M. Effects of Increasing pH on Nitrous Oxide and Dinitrogen Emissions from Denitrification in Sterilized and Unsterilized Forest Soils. Forests. 2022; 13(10):1589. https://doi.org/10.3390/f13101589
Chicago/Turabian StyleWang, Yajing, Wenchao Cao, Jingheng Guo, and Minghu Zhang. 2022. "Effects of Increasing pH on Nitrous Oxide and Dinitrogen Emissions from Denitrification in Sterilized and Unsterilized Forest Soils" Forests 13, no. 10: 1589. https://doi.org/10.3390/f13101589
APA StyleWang, Y., Cao, W., Guo, J., & Zhang, M. (2022). Effects of Increasing pH on Nitrous Oxide and Dinitrogen Emissions from Denitrification in Sterilized and Unsterilized Forest Soils. Forests, 13(10), 1589. https://doi.org/10.3390/f13101589