Comparison of the Foraging Activity of Bats in Coniferous, Mixed, and Deciduous Managed Forests
Abstract
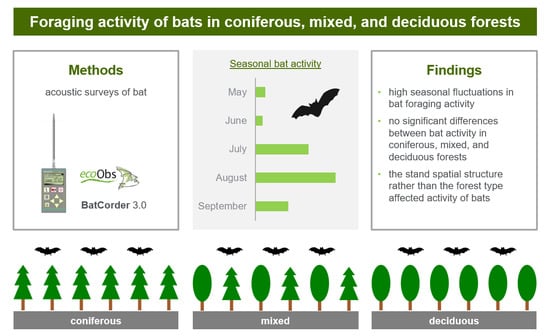
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Bat Acoustic Surveys
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rodríguez-San Pedro, A.; Simonetti, J.A. The relative influence of forest loss and fragmentation on insectivorous bats: Does the type of matrix matter? Landsc. Ecol. 2015, 30, 1561–1572. [Google Scholar] [CrossRef]
- Vasko, V.; Blomberg, A.S.; Vesterinen, E.J.; Suominen, K.M.; Ruokolainen, L.; Brommer, J.E.; Norrdahl, K.; Niemelä, P.; Laine, V.N.; Selonen, V.; et al. Within-season changes in habitat use of forest-dwelling boreal bats. Ecol. Evol. 2020, 10, 4164–4174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langridge, J.; Pisanu, B.; Laguet, S.; Archaux, F.; Tillon, L. The role of complex vegetation structures in determining hawking bat activity in temperate forests. For. Ecol. Manag. 2019, 448, 559–571. [Google Scholar] [CrossRef]
- Brigham, R.M. Bats in forests: What we know and what we need to learn. In Bats in Forests: Conservation and Management; Lacki, M.J., Hayes, J.P., Kurta, A., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2007; pp. 1–15. [Google Scholar]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Böhm, S.M.; Wells, K.; Kalko, E.K.V. Top-down control of herbivory by birds and bats in the canopy of temperate broad-leaved oaks (Quercus robur). PLoS ONE 2011, 6, e17857. [Google Scholar] [CrossRef] [PubMed]
- Lacki, M.J.; Johnson, J.S.; Dodd, L.E.; Baker, M.D. Prey consumption of insectivorous bats in coniferous forests of north-central Idaho. Northwest Sci. 2007, 81, 199–205. [Google Scholar] [CrossRef]
- Krusic, R.A.; Yamasaki, M.; Neefus, C.; Pekins, P. Bat habitat use in White Mountain National Forest. J. Wildl. Manag. 1996, 60, 625–631. [Google Scholar] [CrossRef]
- Grindal, S.D.; Brigham, R.M. Impacts of forest harvesting on habitat use by foraging insectivorous bats at different spatial scales. Ecoscience 1999, 6, 25–34. [Google Scholar] [CrossRef]
- Jung, T.S.; Thompson, I.D.; Titman, R.D.; Applejohn, A.P. Habitat selection by forest bats in relation to mixed-wood stand types and structure in central Ontario. J. Wildl. Manag. 1999, 63, 1306–1319. [Google Scholar] [CrossRef]
- Loeb, S.C.; O’Keefe, J.M. Habitat use by forest bats in South Carolina in relation to local, stand, and landscape characteristics. J. Wildl. Manag. 2006, 70, 1210–1218. [Google Scholar] [CrossRef]
- Russo, D.; Jones, G. Use of foraging habitats by bats in a Mediterranean area determined by acoustic surveys: Conservation implications. Ecography 2003, 26, 197–209. [Google Scholar] [CrossRef]
- Erickson, J.L.; West, S.D. Associations of bats with local structure and landscape features of forested stands in western Oregon and Washington. Biol. Conserv. 2003, 109, 95–102. [Google Scholar] [CrossRef]
- Crampton, L.H.; Barclay, R.M.R. Selection of roosting and foraging habitat by bats in different-aged aspen mixedwood stands. Conserv. Biol. 1998, 12, 1347–1358. [Google Scholar] [CrossRef]
- Bender, M.J.; Perea, S.; Castleberry, S.B.; Miller, D.A.; Wigley, T.B. Influence of insect abundance and vegetation structure on site-occupancy of bats in managed pine forests. For. Ecol. Manag. 2021, 482, 118839. [Google Scholar] [CrossRef]
- Kalcounis, M.C.; Hobson, K.A.; Brigham, R.M.; Hecker, K.R. Bat activity in the boreal forest: Importance of stand type and vertical strata. J. Mammal. 1999, 80, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Russ, J.M.; Montgomery, W.I. Habitat associations of bats in Northern Ireland: Implications for conservation. Biol. Conserv. 2002, 108, 49–58. [Google Scholar] [CrossRef]
- Tibbels, A.E.; Kurta, A. Bat activity is low in thinned and unthinned stands of red pine. Can. J. For. Res. 2003, 33, 2436–2442. [Google Scholar] [CrossRef]
- Rudolph, B.-U.; Liegl, A.; Helversen, O.V. Habitat selection and activity patterns in the greater mouse-eared bat Myotis myotis. Acta Chiropterologica 2009, 11, 351–361. [Google Scholar] [CrossRef]
- Luszcz, T.M.J.; Barclay, R.M.R. Influence of forest composition and age on habitat use by bats in southwestern British Columbia. Can. J. Zool. 2016, 94, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Charbonnier, Y.; Gaüzère, P.; van Halder, I.; Nezan, J.; Barnagaud, J.-Y.; Jactel, H.; Barbaro, L. Deciduous trees increase bat diversity at stand and landscape scales in mosaic pine plantations. Landsc. Ecol. 2016, 31, 291–300. [Google Scholar] [CrossRef]
- Ciechanowski, M. Habitat preferences of bats in anthropogenically altered, mosaic landscapes of northern Poland. Eur. J. Wildl. Res. 2015, 61, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Bontadina, F.; Schmied, S.F.; Beck, A.; Arlettaz, R. Changes in prey abundance unlikely to explain the demography of a critically endangered Central European bat. J. Appl. Ecol. 2008, 45, 641–648. [Google Scholar] [CrossRef]
- Burns, L.K.L.; Loeb, S.C.; Bridges, W.C. Effects of fire and its severity on occupancy of bats in mixed pine-oak forests. For. Ecol. Manag. 2019, 446, 151–163. [Google Scholar] [CrossRef]
- Wermundsen, T.; Siivonen, Y. Foraging habitats of bats in southern Finland. Acta Theriol. 2008, 53, 229–240. [Google Scholar] [CrossRef]
- Lacki, M.J.; Cox, D.R.; Dodd, L.E.; Dickinson, M.B. Response of northern bats (Myotis septentrionalis) to prescribed fires in eastern Kentucky forests. J. Mammal. 2009, 90, 1165–1175. [Google Scholar] [CrossRef] [Green Version]
- Patriquin, K.J.; Barclay, R.M.R. Foraging by bats in cleared, thinned and unharvested boreal forest. J. Appl. Ecol. 2003, 40, 646–657. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.G.; Racey, P.A. Natterer’s bats prefer foraging in broad-leaved woodlands and river corridors. J. Zool. 2008, 275, 314–322. [Google Scholar] [CrossRef]
- Walsh, A.L.; Harris, S. Foraging habitat preferences of vespertilionid bats in Britain. J. Appl. Ecol. 1996, 33, 508–518. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-San Pedro, A.; Simonetti, J.A. Foraging activity by bats in a fragmented landscape dominated by exotic pine plantations in central Chile. Acta Chiropterologica 2013, 15, 393–398. [Google Scholar] [CrossRef]
- Froidevaux, J.S.P.; Barbaro, L.; Vinet, O.; Larrieu, L.; Bas, Y.; Molina, J.; Calatayud, F.; Brin, A. Bat responses to changes in forest composition and prey abundance depend on landscape matrix and stand structure. Sci. Rep. 2021, 11, 10586. [Google Scholar] [CrossRef]
- O’Keefe, J.M.; Loeb, S.C.; Hill, H.S., Jr.; Lanham, J.D. Quantifying clutter: A comparison of four methods and their relationship to bat detection. For. Ecol. Manag. 2014, 322, 1–9. [Google Scholar] [CrossRef]
- Patriquin, K.J.; Hogberg, L.K.; Chruszcz, B.J.; Barclay, R.M.R. The influence of habitat structure on the ability to detect ultrasound using bat detectors. Wildl. Soc. Bull. 2003, 31, 475–481. [Google Scholar]
- Thomas, D.W. The distribution of bats in different ages of Douglas-fir forest. J. Wildl. Manag. 1988, 52, 619–626. [Google Scholar] [CrossRef]
- Węgiel, A.; Grzywiński, W.; Ciechanowski, M.; Jaros, R.; Kalcounis-Rüppell, M.; Kmiecik, A.; Kmiecik, P.; Węgiel, J. The foraging activity of bats in managed pine forests of different ages. Eur. J. For. Res. 2019, 138, 383–396. [Google Scholar] [CrossRef] [Green Version]
- Campbell, L.A.; Hallett, J.G.; O’Connell, M.A. Conservation of bats in managed forests: Use of roosts by Lasionycteris noctivagans. J. Mammal. 1996, 77, 976–984. [Google Scholar] [CrossRef] [Green Version]
- Kalcounis-Rüppell, M.C.; Psyllakis, J.M.; Brigham, R.M. Tree roost selection by bats: An empirical synthesis using meta-analysis. Wildl. Soc. Bull. 2005, 33, 1123–1132. [Google Scholar] [CrossRef]
- Müller, J.; Mehr, M.; Bässler, C.; Fenton, M.B.; Hothorn, T.; Pretzsch, H.; Klemmt, H.-J.; Brandl, R. Aggregative response in bats: Prey abundance versus habitat. Oecologia 2012, 169, 673–684. [Google Scholar] [CrossRef]
- Hagar, J.C. Wildlife species associated with non-coniferous vegetation in Pacific Northwest conifer forests: A review. For. Ecol. Manag. 2007, 246, 108–122. [Google Scholar] [CrossRef]
- Klapwijk, M.J.; Björkman, C. Mixed forests to mitigate risk of insect outbreaks. Scand. J. For. Res. 2018, 33, 772–780. [Google Scholar] [CrossRef]
- Randall, L.A.; Barclay, R.M.R.; Reid, M.L.; Jung, T.S. Recent infestation of forest stands by spruce beetles does not predict habitat use by little brown bats (Myotis lucifugus) in southwestern Yukon, Canada. For. Ecol. Manag. 2011, 261, 1950–1956. [Google Scholar] [CrossRef]
- Woś, A. Climate of Poland [Klimat Polski]; PWN Scientific Publishers: Warszawa, Poland, 1999; p. 301. [Google Scholar]
- Russ, J. British Bat Calls: A Guide to Species Identification; Pelagic Publishing Ltd.: London, UK, 2012. [Google Scholar]
- Runkel, V.; Gerding, G.; Marckmann, U. The Handbook of Acoustic Bat Detection; Pelagic Publishing: Exeter, UK, 2021. [Google Scholar]
- Ahlén, I. Identification of Bats in Flight; Swedish Society for Conservation of Nature & Swedish Youth Association for Environmental Studies and Conservation: Stockholm, Sweden, 1990; p. 50. [Google Scholar]
- Barataud, M. Acoustic ecology of European bats. In Species Identification and Studies of Their Habitats and Foraging Behaviour; Biotope Editions, Mèze; National Museum of Natural History: Paris, France, 2015; p. 352. [Google Scholar]
- Menzel, J.M.; Menzel, M.A.; Kilgo, J.C.; Ford, W.M.; Edwards, J.W.; McCracken, G.F. Effect of habitat and foraging height on bat activity in the Coastal Plain of South Carolina. J. Wildl. Manag. 2005, 69, 235–245. [Google Scholar] [CrossRef]
- Ford, W.M.; Menzel, J.M.; Menzel, M.A.; Edwards, J.W.; Kilgo, J.C. Presence and absence of bats across habitat scales in the Upper Coastal Plain of South Carolina. J. Wildl. Manag. 2006, 70, 1200–1209. [Google Scholar] [CrossRef]
- Kirkpatrick, L.; Maher, S.J.; Lopez, Z.; Lintott, P.R.; Bailey, S.A.; Dent, D.; Park, K.J. Bat use of commercial coniferous plantations at multiple spatial scales: Management and conservation implications. Biol. Conserv. 2017, 206, 1–10. [Google Scholar] [CrossRef]
- Fuentes-Montemayor, E.; Goulson, D.; Cavin, L.; Wallace, J.M.; Park, K.J. Fragmented woodlands in agricultural landscapes: The influence of woodland character and landscape context on bats and their insect prey. Agric. Ecosyst. Environ. 2013, 172, 6–15. [Google Scholar] [CrossRef]
- Ruczyński, I.; Nicholls, B.; MacLeod, C.D.; Racey, P.A. Selection of roosting habitats by Nyctalus noctula and Nyctalus leisleri in Bialowieza Forest-Adaptive response to forest management? For. Ecol. Manag. 2010, 259, 1633–1641. [Google Scholar] [CrossRef]
- Cisneros, L.M.; Fagan, M.E.; Willig, M.R. Effects of human-modified landscapes on taxonomic, functional and phylogenetic dimensions of bat biodiversity. Divers. Distrib. 2015, 21, 523–533. [Google Scholar] [CrossRef]
- Klingbeil, B.T.; Willig, M.R. Seasonal differences in population-, ensemble- and community-level responses of bats to landscape structure in Amazonia. Oikos 2010, 119, 1654–1664. [Google Scholar] [CrossRef]
- Pereira, M.J.R.; Peste, F.; Paula, A.; Pereira, P.; Bernardino, J.; Vieira, J.; Bastos, C.; Mascarenhas, M.; Costa, H.; Fonseca, C. Managing coniferous production forests towards bat conservation. Wildl. Res. 2016, 43, 80–92. [Google Scholar] [CrossRef]
- Akasaka, T.; Nakano, D.; Nakamura, F. Influence of prey variables, food supply, and river restoration on the foraging activity of Daubenton’s bat (Myotis daubentonii) in the Shibetsu River, a large lowland river in Japan. Biol. Conserv. 2009, 142, 1302–1310. [Google Scholar] [CrossRef]
- Loeb, S.C. Qualitative synthesis of temperate bat responses to silvicultural treatments—Where do we go from here? J. Mammal. 2020, 101, 1513–1525. [Google Scholar] [CrossRef]
- Ciechanowski, M. Utilization of artificial shelters by bats (Chiroptera) in three different types of forest. Folia Zool. 2005, 54, 31–37. [Google Scholar]
- Rueegger, N. Variation in summer and winter microclimate in multi-chambered bat boxes in Eastern Australia: Potential eco-physiological implications for bats. Environments 2019, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Deeley, S.; Ford, W.M.; Kalen, N.J.; Freeze, S.R.; St. Germain, M.; Muthersbaugh, M.; Barr, E.; Kniowski, A.; Silvis, A.; De La Cruz, J. Mid-Atlantic Big Brown and Eastern Red Bats: Relationships between Acoustic Activity and Reproductive Phenology. Diversity 2022, 14, 319. [Google Scholar] [CrossRef] [PubMed]




| Sample Plot | Species Composition of the Stand | Age (Years) | Stand Density (Trees per Hectare) | Mean DBH ± SD (cm) | Mean Tree Height ± SD (m) | Basal Area (m2·ha−1) | Stand Volume (m3·ha−1) |
|---|---|---|---|---|---|---|---|
| PINE-1 | pine-100% | 77 | 322 | 35.2 ± 7.0 | 31.1 ± 3.0 | 32.5 | 450 |
| PINE-2 | pine-100% | 75 | 245 | 35.2 ± 7.1 | 30.2 ± 1.9 | 24.8 | 334 |
| MIX-1 | oak-77%, pine-23% | 122 | 170 | 38.0 ± 7.4 | 28.6 ± 3.0 | 20.0 | 284 |
| MIX-2 | pine-76%, oak-24% | 102 | 308 | 36.4 ± 8.8 | 25.5 ± 3.2 | 33.9 | 404 |
| OAK-1 | oak-100% | 127 | 168 | 41.7 ± 9.6 | 26.9 ± 2.1 | 24.2 | 350 |
| OAK-2 | oak-100% | 122 | 165 | 38.3 ± 7.8 | 26.7 ± 2.0 | 19.8 | 280 |
| No | Bat Species/Genus or Sonotype Group | Nights with Bat Passes | Number of Bat Passes | |||
|---|---|---|---|---|---|---|
| PINE | MIX | OAK | Total | |||
| 1 | Pipistrellus pygmaeus | 107 | 616 | 726 | 668 | 2010 |
| 2 | Nyctaloid (Nyctalus sp./Eptesicus sp./Vespertilio murinus) | 85 | 257 | 360 | 151 | 768 |
| 3 | Nyctalus noctula | 80 | 179 | 233 | 54 | 466 |
| 4 | Myotis sp. | 77 | 69 | 133 | 59 | 261 |
| 5 | Pipistrellus nathusii | 76 | 142 | 34 | 80 | 256 |
| 6 | Unidentified bat species | 43 | 19 | 36 | 185 | 240 |
| 7 | Mkm (Myotis daubentonii/M. mystacinus/M. brandtii/M. bechsteinii) | 78 | 45 | 77 | 68 | 190 |
| 8 | Pipistrellus sp. | 44 | 70 | 57 | 51 | 178 |
| 9 | Pipistrellus pipistrellus | 28 | 11 | 16 | 26 | 53 |
| 10 | Barbastella barbastellus | 17 | 4 | 4 | 25 | 33 |
| 11 | Nycmi (Nyctalus leisleri/Eptesicus serotinus/Vespertilio murinus) | 14 | 6 | 6 | 7 | 19 |
| 12 | Eptesicus serotinus | 11 | 6 | 4 | 6 | 16 |
| 13 | Myotis daubentonii | 6 | 1 | 6 | 1 | 8 |
| 14 | Mbart (Myotis mystacinus/brandtii) | 6 | 3 | 0 | 5 | 8 |
| 15 | Myotis myotis | 8 | 1 | 7 | 0 | 8 |
| 16 | Plecotus sp. | 4 | 1 | 0 | 5 | 6 |
| TOTAL | 150 | 1430 | 1699 | 1391 | 4520 | |
| Month (Phenological Season) | Mean (±SD) Number of Bat Passes per Night | ||
|---|---|---|---|
| Coniferous | Mixed | Deciduous | |
| May (gestation) | 9.2 ± 12.7 c | 8.8 ± 4.4 b | 6.7 ± 3.6 bc |
| June (births) | 4.0 ± 4.9 c | 11.0 ± 14.0 b | 2.7 ± 3.1 c |
| July (lactation) | 43.6 ± 18.3 b | 62.4 ± 23.0 a | 28.5 ± 17.6 b |
| August (mating/dispersion) | 84.3 ± 11.8 a | 51.0 ± 10.7 a | 69.6 ± 27.5 a |
| September (mating/dispersion) | 8.2 ± 11.7 c | 43.9 ± 17.3 a | 31.6 ± 27.6 b |
| Variable | Source | Chisq | Df | p |
|---|---|---|---|---|
| All bats | Forest type | 3.4581 | 2 | 0.1774 |
| Temperature | 0.0315 | 1 | 0.8592 | |
| Wind speed | 8.3324 | 1 | 0.0039 ** | |
| DBH | 0.9410 | 1 | 0.3320 | |
| Myotis | Forest type | 39.5001 | 2 | <0.0001 *** |
| Temperature | 7.1529 | 1 | 0.0075 ** | |
| Wind speed | 45.5172 | 1 | <0.0001 *** | |
| DBH | 0.1134 | 1 | 0.7363 | |
| Pipistrellus | Forest type | 90.6523 | 2 | <0.0001 *** |
| Temperature | 1.9225 | 1 | 0.1656 | |
| Wind speed | 1.2691 | 1 | 0.2599 | |
| DBH | 105.7953 | 1 | <0.0001 *** | |
| Nyctalus | Forest type | 44.4751 | 2 | <0.0001 *** |
| Temperature | 10.3061 | 1 | 0.0013 ** | |
| Wind speed | 1.0493 | 1 | 0.3057 | |
| DBH | 2.3368 | 1 | 0.1263 |
| Forest Type | All Bats | Myotis | Pipistrellus | Nyctalus |
|---|---|---|---|---|
| MIX | 3.25 (2.38; 4.13) | 1.54 (1.09; 2.00) a | 2.26 (1.18; 3.35) b | 1.74 (0.01; 3.47) a |
| OAK | 2.85 (1.92; 3.77) | 1.07 (0.56; 1.58) b | 1.63 (0.54; 2.72) c | 0.38 (−1.38; 2.13) b |
| PINE | 3.18 (2.26; 4.09) | 0.89 (0.38; 1.39) b | 2.71 (1.63; 3.80) a | 1.56 (−0.18; 3.31) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Węgiel, A.; Grzywiński, W.; Jaros, R.; Łacka, A.; Węgiel, J. Comparison of the Foraging Activity of Bats in Coniferous, Mixed, and Deciduous Managed Forests. Forests 2023, 14, 481. https://doi.org/10.3390/f14030481
Węgiel A, Grzywiński W, Jaros R, Łacka A, Węgiel J. Comparison of the Foraging Activity of Bats in Coniferous, Mixed, and Deciduous Managed Forests. Forests. 2023; 14(3):481. https://doi.org/10.3390/f14030481
Chicago/Turabian StyleWęgiel, Andrzej, Witold Grzywiński, Radosław Jaros, Agnieszka Łacka, and Jolanta Węgiel. 2023. "Comparison of the Foraging Activity of Bats in Coniferous, Mixed, and Deciduous Managed Forests" Forests 14, no. 3: 481. https://doi.org/10.3390/f14030481
APA StyleWęgiel, A., Grzywiński, W., Jaros, R., Łacka, A., & Węgiel, J. (2023). Comparison of the Foraging Activity of Bats in Coniferous, Mixed, and Deciduous Managed Forests. Forests, 14(3), 481. https://doi.org/10.3390/f14030481