Forest Site Classification in the Southern Andean Region of Ecuador: A Case Study of Pine Plantations to Collect a Base of Soil Attributes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Forest Attributes
2.3. Enviromental Variables
2.3.1. Topographical and Climatic Attributes
2.3.2. Soil Attributes
Soil Sampling
Physical and Chemical Soil Attributes
2.4. Statistical Analyses
- (a)
- Reduction of the dimensionality of soil chemical dataDue to the large number of soil variables (see Table 2), data reduction was necessary, mainly in the soil chemical data because of their collinearity. The non-parametric technique of Classification and Regression Trees (CART) [60] was applied for the reduction of the dimensionality. CART is a less sensitive method to collinearity [61], is very useful in variable selection by accounting for the attribute importance [62], and is suitable for the analysis of unbalanced ecological data containing nonlinear relationships and missing values [63]. CART was applied using the height of dominant trees (DH) at a reference age of 20 years as a response variable. The height of dominant trees at a reference age has been used in our study because it is the most common indicator to measure site productivity in even-aged stands and therefore is an index of forest productivity widely used in forestry [64,65]. It is recommended for studies of site classification and stand productivity [66], basically because it is less dependent on stand density and thinning [67]. The DH data per plot was standardized at 20 years (DH20) using a linear model based on the criteria of the mean annual increment (MAI) of tree height [39]. This linear standardization was used because the growth curve for pine (Pinus sylvestris) is approximately linear [68] in the considered growth interval between 14 and 22 years.
- (b)
- Generation of forests site classesForest site classification was developed using Cluster and Partitioning Analysis [69]. A cluster analysis using the Ward’s criterion was carried out with a database of the selected soil chemical variables by CART and the soil morphological and physical (see Table 2), climate, and topographical variables. Cluster and Partitioning Analysis was applied because it is a non-parametric method that has been used in several studies directly related to the generation of forest site classes and vegetation [28,29,70].
- (c)
- Identification and assessment of the main relationships between environmental variables and forest productivity.Partial Least Squares (PLS) regression was applied to identify the strength of the relationship between the environmental variables and forest site classification. PLS-regression analysis was used due to its capacity to work with strongly correlated data, noisy variables, missing values, and a larger number of variables than the sample size [71,72]. The standardized DH20 was also used to apply the PLS-regression and the validation of the PLS was developed by a K-fold cross-validation [73], and the final number of components was selected by a permutation test [74]. In the PLS regression, the importance of a variable in the relationship with the DH20 was determined by the use of a filter method based on a measure of variable importance in partial least projections known as variable importance in projection (VIP) [75]. Finally, Spearman correlation analysis was used to describe the relationships of the selected variables after the PLS-regression.
3. Results
3.1. Characterization of Environmental Variables
3.1.1. Climate and Topography
3.1.2. Soils
Physico-Chemical Soil Properties
Soil Nutrient Stocks
3.1.3. Forest Stand Characteristics
3.2. Forest Site Classification
3.3. Basic Protocol for Forest Site Classification in the South Andes
4. Discussion
4.1. Effects of Climate and Topography on Forest Productivity
4.2. Effects of Soils on Forest Productivity
4.3. Attributes Useful in a Future Protocol for Site Classification
4.4. Use of Forest Site Classification towards a Sustainable Silviculture in Ecuador
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sloan, S.; Sayer, J.A. Forest Resources Assessment of 2015 shows positive global trends but forest loss and degradation persist in poor tropical countries. For. Ecol. Manag. 2015, 352, 134–145. [Google Scholar] [CrossRef]
- Mosandl, R.; Günter, S.; Stimm, B.; Weber, M. Ecuador suffers the highest deforestation rate in South America. In Gradients in a Tropical Mountain Ecosystem of Ecuador SE-4; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2008; Volume 198, pp. 37–40. [Google Scholar]
- Evans, J. Planted Forests: Uses, Impacts and Sustainability; CAB International and FAO: Rome, Italy, 2009. [Google Scholar]
- Brandbyge, J. Reforestación de los Andes Ecuatorianos con Especies Nativas; CESA-Intercooperation Suiza: Quito, Ecuador, 1991. [Google Scholar]
- Farley, K.A. Grasslands to tree plantations: Forest transition in the Andes of Ecuador. Ann. Assoc. Am. Geogr. 2007, 97, 755–771. [Google Scholar] [CrossRef]
- Van Voss, O.; Aguirre, N.; Hofstede, R. Sistemas Forestales Integrales Para la Sierra del Ecuador; ABYA-YALA: Quito, Ecuador, 2001. [Google Scholar]
- Knoke, T.; Bendix, J.; Pohle, P.; Hamer, U.; Hildebrandt, P.; Roos, K.; Gerique, A.; Sandoval, M.L.; Breuer, L.; Tischer, A.; et al. Afforestation or intense pasturing improve the ecological and economic value of abandoned tropical farmlands. Nat. Commun. 2014, 5, 5612. [Google Scholar] [CrossRef] [PubMed]
- Mejía, E.; Pacheco, P. Aprovechamiento Forestal y Mercados de la Madera en la Amazonía Ecuatoriana; CIFOR: Bogor, Indonesia, 2013. [Google Scholar]
- Ministerio del Ambiente de Ecuador. Plan Nacional de Forestación y Reforestación; Ministerio del Ambiente de Ecuador: Quito, Ecuador, 2006. [Google Scholar]
- Buytaert, W.; Iñiguez, V.; De Bièvre, B. The effects of afforestation and cultivation on water yield in the Andean páramo. For. Ecol. Manag. 2007, 251, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Chacón, G.; Gagnon, D.; Paré, D. Comparison of soil properties of native forests, Pinus patula plantations and adjacent pastures in the Andean highlands of southern Ecuador: Land use history or recent vegetation effects? Soil Use Manag. 2009, 25, 427–433. [Google Scholar] [CrossRef]
- Farley, K.A.; Kelly, E.F. Effects of afforestation of a páramo grassland on soil nutrient status. For. Ecol. Manag. 2004, 195, 281–290. [Google Scholar] [CrossRef]
- Quichimbo, P.; Tenorio, G.; Borja, P.; Cárdenas, I.; Crespo, P.; Célleri, R. Efectos sobre las propiedades físicas y químicas de los suelos por el cambio de la cobertura vegetal y uso del suelo: Páramo de Quimsacocha al sur del Ecuador. Suelos Ecuatoriales 2012, 42, 138–153. [Google Scholar]
- Leischner, B.; Bussmann, R.W. Mercado y uso de madera en el Sur de Ecuador. Lyonia 2003, 5, 51–60. [Google Scholar]
- Buytaert, W.; Cuesta-Camacho, F.; Tobón, C. Potential impacts of climate change on the environmental services of humid tropical alpine regions. Glob. Ecol. Biogeogr. 2011, 20, 19–33. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Global Forest Resources Assessment 2000; Main Report; Forestry Paper 140; FAO: Rome, Italy, 2000; Volume 20. [Google Scholar]
- Günter, S.; Gonzalez, P.; Álvarez, G.; Aguirre, N.; Palomeque, X.; Haubrich, F.; Weber, M. Determinants for successful reforestation of abandoned pastures in the Andes: Soil conditions and vegetation cover. For. Ecol. Manag. 2009, 258, 81–91. [Google Scholar] [CrossRef]
- Mason, W.L. Multiple-use silviculture in temperate plantation forestry. In Enciclopedia of Forest Sciences; Burley, J., Evans, J., Youngquist, J., Eds.; Elsevier Ltd.: Oxford, UK, 2004; Volume 2, pp. 859–865. ISBN 0-12-145160-7. [Google Scholar]
- Louw, J.H.; Scholes, M. Forest site classification and evaluation: A South African perspective. For. Ecol. Manag. 2002, 171, 153–168. [Google Scholar] [CrossRef]
- Ebeling, J.; Yasué, M. The effectiveness of market-based conservation in the tropics: Forest certification in Ecuador and Bolivia. J. Environ. Manag. 2009, 90, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Colmenares Zapata, A.J. Influencia del PNUMA en la redefinición de las políticas públicas forestales del Ecuador, 2008–2014. Estado Comunes 2017, 2, 23–36. [Google Scholar]
- Mohebalian, P.M.; Aguilar, F.X. Forest policy and economics additionality and design of forest conservation programs: Insights from Ecuador’s Socio Bosque Program. For. Policy Econ. 2016, 71, 103–114. [Google Scholar] [CrossRef]
- Dercon, G.; Bossuyt, B.; Bievre, B.; Cisneros, F.; Deckers, J. Zonificacion Agroecologica del Austro Ecuatoriano; U Ediciones: Cuenca, Ecuador, 1998. [Google Scholar]
- Bydekerke, L.; Van Ranst, E.; Vanmechelen, L.; Groenemans, R. Land suitability assessment for cherimoya in southern Ecuador using expert knowledge and GIS. Agric. Ecosyst. Environ. 1998, 69, 89–98. [Google Scholar] [CrossRef]
- Bontemps, J.D.; Bouriaud, O. Predictive approaches to forest site productivity: Recent trends, challenges and future perspectives. Forestry 2014, 87, 109–128. [Google Scholar] [CrossRef]
- Corns, I. Forest site classification in Alberta: Its evolution and present status. For. Chron. 1992, 68, 85–93. [Google Scholar] [CrossRef]
- Barnes, B.; Zak, D.; Denton, S.; Spurr, S. Forest Ecology, 4th ed.; Wiley: New York, NY, USA, 1997. [Google Scholar]
- Wu, Y.; Qin, K.; Zhang, M.; Li, M. Study on forest site classification of southern Xiaoxing’an Mountain in northeast of China. World Rural Obs. 2013, 5, 27–32. [Google Scholar]
- Li, S.; Shen, G.; Zhai, M.; Li, J. Quantitative site classification in the key county in the conversion of farmland to forests project. Front. For. China 2006, 1, 157–162. [Google Scholar]
- Lukac, M.; Godbold, D. Soil Ecology in Northern Forests: A Belowground View of a Changing World; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Osman, K. Forest Soils: Properties and Management; Springer: Cham, Switzerland, 2013. [Google Scholar]
- Pokharel, B.; Dech, J.P. An ecological land classification approach to modeling the production of forest biomass. For. Chron. 2011, 87, 23–32. [Google Scholar] [CrossRef]
- Morris, L.A. Nutrient cycling. In Enciclopedia of Forest Sciences; Burley, J., Evans, J., Youngquist, J., Eds.; Elsevier Ltd.: Oxford, UK, 2004; Volume 3, pp. 1227–1235. ISBN 0-12-145160-7. [Google Scholar]
- Liess, M.; Glaser, B.; Huwe, B. Digital soil mapping in Southern Ecuador. Erdkunde 2009, 63, 309–319. [Google Scholar] [CrossRef]
- Breckle, S.; Breckle, U.; Homeier, J.; Scheffer, A. Mineral deficiencies in a pine plantation in southern Ecuador. Ecotropica 2005, 11, 79–85. [Google Scholar]
- Larrea, C.; Carrasco, F.; Cervantes, J.; Viedma, N. INFOPLAN, Atlas para el Desarrollo Local; ODEPLAN Presidencia de la República, Secretaría General de la Presidencia, CONAM y COSUDE: Quito, Ecuador, 1999. [Google Scholar]
- Alianza Jatun Sacha-CDC (Corporación Centro de Datos para la Conservación). Almanaque Electrónico Ecuatoriano 2003; Alianza Jatun Sacha-CDC: Quito, Ecuador, 2003. [Google Scholar]
- Inerhi-Predesur-Conade. Plan Integral de Desarrollo de los Recursos Hídricos de la Provincia de Loja; Departamento de Desarrollo Regional y Medio Ambiente: Washington, DC, USA, 1994. [Google Scholar]
- West, P.W. Tree and Forest Measurement, 2nd ed.; Biomedical and Life Sciences; Springer: Heidelberg, Germany, 2009. [Google Scholar]
- Curtis, R.O.; Marshall, D.D. Why quadratic mean diameter? West. J. Appl. For. 2000, 15, 137–139. [Google Scholar]
- McElhinny, C.; Gibbons, P.; Brack, C. An objective and quantitative methodology for constructing an index of stand structural complexity. For. Ecol. Manag. 2006, 235, 54–71. [Google Scholar] [CrossRef]
- Zevenbergen, L.W.; Thorne, C.R. Quantitative analysis of land surface topography. Earth Surf. Processes Landf. 1987, 12, 47–56. [Google Scholar] [CrossRef]
- Conrad, O. Entwurf, Funktionsumfang und Anwendung eines Systems für Automatisierte Geowissenschaftliche Analysen; Georg-August-Universität Göttingen: Göttingen, Germany, 2006. [Google Scholar]
- QGIS Development Team. QGIS 2.18.9 “Las Palmas”; Open Source Geospatial Foundation: Beaverton, OR, USA, 2016. [Google Scholar]
- Alianza Jatun Sacha-CDC (Corporación Centro de Datos para la Conservación). Informe Final Proyecto AG-CT-011: Sistemas de Información Geográfica para Aplicaciones Agropecuarias en el Ordenamiento de Territorios y Manejo Integral de Cuencas; Alianza Jatun Sacha-CDC: Quito, Ecuador, 2003. [Google Scholar]
- Fries, A.; Rollenbeck, R.; Nauß, T.; Peters, T.; Bendix, J. Near surface air humidity in a megadiverse Andean mountain ecosystem of southern Ecuador and its regionalization. Agric. For. Meteorol. 2012, 152, 17–30. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Guidelines for Soil Description, 4th ed.; Jahn, R., Blume, H., Asio, V., Spaargaren, O., Schad, P., Eds.; FAO: Rome, Italy, 2006. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Vesterdal, L.; Schmidt, I.K.; Callesen, I.; Nilsson, L.O.; Gundersen, P. Carbon and nitrogen in forest floor and mineral soil under six common European tree species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
- Wolff, B.; Riek, W. Deutscher Waldbodenbericht 1996. Er-Gebnisse der Bundesweiten Bodenzustandserhebung im Wald von 1987–1993 (BZE) Bd. 1 und 2.; Bundesministerium für Ernährung, Landwirtschaft und Forsten: Bonn, Germany, 1997. [Google Scholar]
- Blume, H.-P.; Stahr, K.; Leinweber, P. Bodenkundliches Praktikum: Eine Einführung in Pedologisches Arbeiten für Ökologen, Land- und Forstwirte, und für Geowissenschaftler, 3rd ed.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2011. [Google Scholar]
- Minasny, B.; Hartemink, A.E. Predicting soil properties in the tropics. Earth-Sci. Rev. 2011, 106, 52–62. [Google Scholar] [CrossRef]
- Ad-Hoc-Arbeitsgruppe Boden. Bodenkundliche Kartieranleitung, 5th ed.; Schweizerbart: Hannover, Germany, 2005. [Google Scholar]
- Botula, Y.; Cornelis, W.; Baert, G.; Mafuka, P.; Van Ranst, E. Particle size distribution models for soils of the humid tropics. J. Soils Sediments 2013, 13, 686–698. [Google Scholar] [CrossRef]
- McKeague, J.A.; Day, J.H. Dithionite- and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can. J. Soil Sci. 1966, 46, 13–22. [Google Scholar] [CrossRef]
- Lüer, B.; Böhmer, A. Comparison between percolation and extraction with 1 M NH4Cl solution to determine the effective cation exchange capacity (CECeff) of soils. J. Plant Nutr. Soil Sci. 2000, 163, 555–557. [Google Scholar] [CrossRef]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Kingston, H.M.; Jassie, L.B. Microwave energy for acid decomposition at elevated temperatures and pressures using biological and botanical samples. Anal. Chem. 1986, 58, 2534–2541. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; The Wadsworth and Brooks-Cole Statistics-Probability Series; Chapman & Hall: London, UK, 1984. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carr, G.; Garc, J.R.; Gruber, B.; Lafourcade, B.; Leit, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Wu, X.; Kumar, V.; Ross Quinlan, J.; Ghosh, J.; Yang, Q.; Motoda, H.; McLachlan, G.J.; Ng, A.; Liu, B.; Yu, P.S.; et al. Top 10 algorithms in data mining. Knowl. Inf. Syst. 2008, 14, 1–37. [Google Scholar] [CrossRef]
- De’Ath, G.; Fabricius, K.E. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology 2000, 81, 3178–3192. [Google Scholar] [CrossRef]
- Dănescu, A.; Albrecht, A.T.; Bauhus, J.; Kohnle, U. Geocentric alternatives to site index for modeling tree increment in uneven-aged mixed stands. For. Ecol. Manag. 2017, 392, 1–12. [Google Scholar] [CrossRef]
- Skovsgaard, J.P.; Vanclay, J.K. Forest site productivity: A review of the evolution of dendrometric concepts for even-aged stands. Forestry 2008, 81, 13–31. [Google Scholar] [CrossRef]
- Skovsgaard, J.P. Mensuration: Forest measurements. Encycl. For. Sci. 2004, 2, 550–566. [Google Scholar]
- Pretzsch, H. Forest Dynamics, Growth and Yield: From Measurement to Model; Springer: Freising, Germany, 2009. [Google Scholar]
- Palahí, M.; Tomé, M.; Pukkala, T.; Trasobares, A.; Montero, G. Site index model for Pinus sylvestris in north-east Spain. For. Ecol. Manag. 2004, 187, 35–47. [Google Scholar] [CrossRef]
- Husson, F.; Le, S.; Pagès, J. Exploratory Multivariate Analysis by Example Using R; Chapman & Hall/CRC Computer Science & Data Analysis; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Serra, J.; Cristobal, J.; Ninyerola, M. A classification procedure for mapping topo-climatic conditions for strategic vegetation planning. Environ. Model. Asses. 2011, 16, 77–89. [Google Scholar] [CrossRef]
- Abdi, H. Partial least squares regression and projection on latent structure regression. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Mevik, B.-H.; Cederkvist, H.R. Mean squared error of prediction (MSEP) estimates for principal component regression (PCR) and partial least squares regression (PLSR). J. Chemom. 2004, 18, 422–429. [Google Scholar] [CrossRef]
- Van der Voet, H. Comparing the predictive accuracy of models using a simple randomization test. Chemom. Intell. Lab. Syst. 1994, 25, 313–323. [Google Scholar] [CrossRef]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Sæbø, S. A review of variable selection methods in Partial Least Squares Regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing 2017; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Aertsen, W.; Kint, V.; Muys, B.; Van Orshoven, J. Effects of scale and scaling in predictive modelling of forest site productivity. Environ. Model. Softw. 2012, 31, 19–27. [Google Scholar] [CrossRef]
- Barnes, B.; Pregitzer, K. Ecological forest site classification. J. For. 1982, 80, 493–498. [Google Scholar]
- Scolforo, J.R.S.; Maestri, R.; Ferraz Filho, A.C.; de Mello, J.M.; de Oliveira, A.D.; de Assis, A.L. Dominant height model for site classification of eucalyptus grandis incorporating climatic variables. Int. J. For. Res. 2013, 2013, 139236. [Google Scholar]
- Huss, J. Stand establishment, treatment and promotion—European experience. In Enciclopedia of Forest Sciences; Burley, J., Evans, J., Youngquist, J., Eds.; Elsevier Ltd.: Oxford, UK, 2004; Volume 1, pp. 14–27. ISBN 0-12-145160-7. [Google Scholar]
- Pojar, J.; Klinka, K.; Meidinger, D. Biogeoclimatic ecosystem classification in British Columbia. For. Ecol. Manag. 1987, 22, 119–154. [Google Scholar] [CrossRef]
- Panferov, O.; Kreilein, H.; Meesenburg, H.; Eichhorn, J.; Gravenhorst, G. Climatic condition at three beech forest sites in central Germany. In Functioning and Management of European Beech Ecosystems; Brumme, B., Khanna, P., Eds.; Springer: Berlin, Germany, 2009; pp. 13–32. [Google Scholar]
- PNUMA; Ilustre Municipio de Loja; Naturaleza y Cultura Internacional. Perspectivas del Medio Ambiente Urbano: GEO Loja; GEO Loja: Loja, Ecuador, 2007. [Google Scholar]
- Kai, Y.; Ying, M.; Huiyan, G.; Peng, L. Site classification of the eastern forest region of Daxing’an Mountains. J. For. Res. 1999, 10, 129–131. [Google Scholar] [CrossRef]
- Wilson, S.; Pyatt, D. The use of ground vegetation and humus type as indicators of soil nutrient regime for an ecological site classification of British forests. For. Ecol. Manag. 2001, 140, 101–116. [Google Scholar] [CrossRef]
- Kübler, D.; Hildebrandt, P.; Günter, S.; Stimm, B.; Weber, M.; Mosandl, R.; Muñoz, J.; Cabrera, O.; Aguirre, N.; Zeilinger, J.; et al. Assessing the importance of topographic variables for the spatial distribution of tree species in a tropical mountain forest. Erdkunde 2016, 70, 19–47. [Google Scholar] [CrossRef]
- Binkley, D.; Fisher, R. Ecology and Management of Forest Soils, 4th ed.; Wiley-Blackwell: New York, NY, USA, 2013. [Google Scholar]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Chapter 6—Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 135–189. [Google Scholar]
- Robertson, G.P.; Groffman, P.M.; Groffman, P.M. Nitrogen Transformations. In Soil Microbiology, Ecology and Biochemistry, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 421–446. [Google Scholar]
- Clarholm, M.; Skyllberg, U. Translocation of metals by trees and fungi regulates pH, soil organic matter turnover and nitrogen availability in acidic forest soils. Soil Biol. Biochem. 2013, 63, 142–153. [Google Scholar] [CrossRef]
- Moyer-Henry, K.; Silva, I.; Macfall, J.; Johannes, E.; Allen, N.; Goldfarb, B.; Rufty, T. Accumulation and localization of aluminium in root tips of loblolly pine seedlings and the associated ectomycorrhiza Pisolithus tinctorius. Plant Cell Environ. 2005, 28, 111–120. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Ogdahl, M.; Chorover, J.; Chadwick, O.A.; Oleksyn, J.; Zytkowiak, R.; Reich, P.B. Tree species effects on soil organic matter dynamics: The role of soil cation composition. Ecosystems 2007, 10, 999–1018. [Google Scholar] [CrossRef]
- Cañadas-Cruz, L. El Mapa Bioclimático y Ecológico del Ecuador; PRONAREG: Quito, Ecuador, 1983. [Google Scholar]
- Yarzábal, L.A.; Chica, E.J.; Quichimbo, P. Microbial diversity of tropical andean soils and low-input sustainable agriculture development. In Agriculturally Important Microbes for Sustainable Agriculture: Volume I: Plant-Soil-Microbe Nexus; Meena, V.S., Mishra, P.K., Bisht, J.K., Pattanayak, A., Eds.; Springer: Singapore, 2017; pp. 207–234. [Google Scholar]
- Poulenard, J.; Podwojewski, P. Alpine soils. In Encyclopedia of Soil Sciences, 2nd ed.; Lal, R., Ed.; CRC Press: Boca Raton, FL, USA, 2006; Volume 1, pp. 75–79. [Google Scholar]
- Gardi, C.; Angelini, M.; Barceló, S.; Comerma, J.; Cruz Gaistardo, C.; Encina Rojas, A.; Jones, A.; Krasilnikov, P.; Santos, M.; Brefin, M.L.; et al. Atlas de Suelos de América Latina y el Caribe; Comisión Europea—Oficina de Publicaciones de la Unión Europea; EUR: Luxembourg, 2014. [Google Scholar]
- Hofstede, R.G.M.; Groenendijk, J.P.; Coppus, R.; Fehse, J.C.; Sevink, J. Impact of pine plantations on soils and vegetation in the Ecuadorian high andes. Mt. Res. Dev. 2002, 22, 159–167. [Google Scholar] [CrossRef]
- Ulloa, J.; Ballari, D.; Campozano, L.; Samaniego, E. Two-step downscaling of Trmm 3b43 V7 precipitation in contrasting climatic regions with sparse monitoring: The case of Ecuador in Tropical South America. Remote Sens. 2017, 9, 758. [Google Scholar] [CrossRef]
- Potthast, K.; Hamer, U.; Makeschin, F. Land-use change in a tropical mountain rainforest region of southern Ecuador affects soil microorganisms and nutrient cycling. Biogeochemistry 2012, 111, 151–167. [Google Scholar] [CrossRef]
- Tischer, A.; Werisch, M.; Döbbelin, F.; Camenzind, T.; Rillig, M.C.; Potthast, K.; Hamer, U. Above- and belowground linkages of a nitrogen and phosphorus co-limited tropical mountain pasture system—Responses to nutrient enrichment. Plant Soil 2015, 391, 333–352. [Google Scholar] [CrossRef]
- Tischer, A.; Blagodatskaya, E.; Hamer, U. Microbial community structure and resource availability drive the catalytic efficiency of soil enzymes under land-use change conditions. Soil Biol. Biochem. 2015, 89, 226–237. [Google Scholar] [CrossRef]
- Beck, E.; Makeschin, F.; Haubrich, F.; Richter, M.; Bendix, J.; Valerezo, C. The Ecosystem (Reserva Biológica San Francisco). In Gradients in a Tropical Mountain Ecosystem of Ecuador; Beck, E., Bendix, J., Kottke, I., Makeschin, F., Mosandl, R., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2008; Volume 198, pp. 1–13. [Google Scholar]
- Onyekwelu, J.; Stimm, B.; Evans, J. Review Plantation Forestry. In Silviculture in the Tropics; Günter, S., Weber, M., Stimm, B., Mosandl, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 399–454. [Google Scholar]
- Corbin, J.D.; Holl, K.D. Applied nucleation as a forest restoration strategy. For. Ecol. Manag. 2012, 265, 37–46. [Google Scholar] [CrossRef]
- Ashton, M. Ecological principles and silvicultural techniques for understanding degradation and restoration of tropical rainforests. In Tropical Ecosystems—Between Protection and Production; Mosandl, R., Weber, M., Stimm, B., Hildebrandt, P., Knoke, T., Eds.; Society for Tropical Ecology: Munich, Germany, 2014; p. 26. [Google Scholar]
- Isbell, F.; Adler, P.; Eisenhauer, N.; Fornara, D.; Kimmel, K.; Kremen, C.; Letourneau, D.; Liebman, M.; Polley, H.; Quijas, S.; et al. Benefits of increasing plant diversity in sustainable agroecosystems. J. Ecol. 2017, 105, 871–879. [Google Scholar] [CrossRef]
- Weber, M.; Stimm, B.; Mosandl, R. Review plantations for protective purposes and rehabilitation. In Silviculture in the Tropics SE-30; Günter, S., Weber, M., Stimm, B., Mosandl, R., Eds.; Tropical Forestry; Springer: Berlin/Heidelberg, Germany, 2011; Volume 8, pp. 475–490. [Google Scholar]
- Haug, I.; Wubet, T.; Weiß, M.; Aguirre, N.; Weber, M.; Günter, S.; Kottke, I. Species-rich but distinct arbuscular mycorrhizal communities in reforestation plots on degraded pastures and in neighboring pristine tropical mountain rain forest. Trop. Ecol. 2010, 51, 125–148. [Google Scholar]
- Aguirre, N.; Günter, S.; Weber, M.; Stimm, B. Enrichment of Pinus patula plantations with native species in southern Ecuador. Lyionia 2006, 10, 33–45. [Google Scholar]
- Mosandl, R.; Günter, S. Sustainable management of tropical mountain forests in Ecuador. In The Tropical Mountain Forest: Patterns and Processes in a Biodiversity Hotspot; Gradstein, S., Homeier, J., Gansert, D., Eds.; Biodiversity and Ecology Series; University of Akron Press: Akron, OH, USA, 2008; pp. 177–193. [Google Scholar]
- Günter, S.; Calvas, B.; Lotz, T.; Bendix, J.; Mosandl, R. Knowledge transfer for conservation and sustainable management of natural resources: A case study from southern Ecuador. In Ecosystem Services, Biodiversity and Environmental Change in a Tropical Mountain Ecosystem of South Ecuador; Bräuning, A., Makeschin, F., Mosandl, R., Scheu, S., Wilcke, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 395–410. [Google Scholar]
- Berthrong, S.T.; Jobbágy, E.G.; Jackson, R.B. A global meta-analysis of soil exchangeable cations, pH, carbon, and nitrogen with afforestation. Ecol. Appl. 2009, 19, 2228–2241. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Luo, Y.; Fang, C.; Chen, J.; Li, B. The effects of plantation practice on soil properties based on the comparison between natural and planted forests: A meta-analysis. Glob. Ecol. Biogeogr. 2012, 21, 318–327. [Google Scholar] [CrossRef]



| Site | ||||
|---|---|---|---|---|
| SAN | ZAM | DOS | VIL | |
| Forest | ||||
| Stand age (years) a | 20 | 18 | 22 | 14 |
| Climate | ||||
| Precipitation (mm year−1) c | 659 | 889 | 839 | 599 |
| Temperature (°C) c | 9.3 | 11.2 | 9.9 | 10.6 |
| Potential Evapotranspiration (mm year−1) c | 1013 | 1053 | 1035 | 1043 |
| Climate (Köppen) e | Humid temperate without dry season | Humid temperate without dry season | Humid temperate without dry season | Humid temperate without dry season |
| Terrain | ||||
| Geology b | Paleozoic metamorphics | Paleozoic metamorphics | Paleozoic metamorphics | Paleozoic metamorphics |
| Relief d | Medium hills | Irregular hillslopes | Irregular hillslopes | Irregular hillslopes |
| Altitude (m a.s.l.) d | 2430 | 2234 | 2402 | 2324 |
| Slope (%) d | 30 | 41 | 41.5 | 44 |
| Aspect d | North | North-West | North-West | North |
| Former land use a | pasture | pasture | pasture | remnant Andean shrubs |
| Factor | Attribute | Measure |
|---|---|---|
| Forest | Dominant height of trees standardized to a plantation age of 20 years (DH20) | m |
| Climate | Precipitation (PRE) | mm year−1 |
| Temperature (TEM) | °C | |
| Potential Evapotranspiration (PET) | mm year−1 | |
| Topography | Slope (SLO) | percentage |
| Aspect (ASP) | slope direction (intercardinal direction) | |
| Altitude (ALT) | m a.s.l. | |
| Soil | Morphological variables: | |
| Maximum root depth (MRD) | cm | |
| Dominant root depth (DRD) | cm | |
| Forest floor thickness (FFT) | cm | |
| Stoniness (STONES) | percentage | |
| Physical variables: | ||
| Bulk density (BD) | Mg m−3 | |
| Granulometry (CLAY, SILT, SAND) | percentage | |
| Chemical variables: | ||
| Base saturation (BS) | percentage | |
| Acidity (pH in water) | pH-value | |
| Soil organic carbon (SOC) | Mg ha−1 | |
| Cation exchange capacity (CEC) | cmol(+) kg−1 | |
| Total nutrients stocks: N, P, K, Ca, Mg, S, Fe, Mn, Zn, Cu, Ni, Na, Al | Mg ha−1 | |
| Plant available nutrient stocks *: P, Na, K, Ca, Mg, Al, Fe, Mn, NH4-N, NO3-N, TIN (Total Inorganic Nitrogen). | kg ha−1 | |
| Total nutrient stocks ratios: N/P, N/K, N/Ca, N/Mg, TIN/P, TIN/K, TIN/Ca, TIN/Mg, C/N. | Ratio (stocks) | |
| Ratios of plant available nutrient stocks */total nutrient stocks: P */P, Na */Na, K */K, Ca */Ca, Mg */Mg, Al */Al, Fe */Fe, Mn */Mn, Ca */Mg * | Ratio (stocks) | |
| Contribution of the available nutrient stocks to the total nutrient stocks | percentage |
| Nutrient (Mg ha−1) | Site | |||
|---|---|---|---|---|
| SAN | ZAM | DOS | VIL | |
| SOC | 11.081 (0.16) b | 26.736 (10.762) a | 18.907 (3.93) ab | 10.765 (4.133) b |
| TN | 0.348 (0.033) b | 0.685 (0.229) a | 0.571 (0.129) a | 0.272 (0.09) b |
| P | 0.028 (0.001) ab | 0.04 (0.017) a | 0.029 (0.013) ab | 0.016 (0.005) b |
| K | 0.076 (0.009) bc | 0.118 (0.062) a | 0.095 (0.028) ab | 0.048 (0.02) c |
| Ca | 0.25 (0.02) a | 0.052 (0.029) a | 0.045 (0.007) a | 0.08 (0.068) a |
| Mg | 0.045 (0.02) a | 0.03 (0.017) a | 0.028 (0.008) a | 0.033 (0.021) a |
| S | 0.028 (0.002) bc | 0.056 (0.021) a | 0.044 (0.009) b | 0.021 (0.008) c |
| Al | 0.452 (0.077) a | 0.251 (0.062) a | 0.249 (0.132) a | 0.169 (0.046) a |
| Fe | 0.183 (0.042) a | 0.102 (0.064) a | 0.163 (0.056) a | 0.086 (0.031) a |
| Mn | 0.045 (0.03) a | 0.008 (0.007) b | 0.021 (0.014) ab | 0.005 (0.004) b |
| Cu | 0.0004 (0) b | 0.0009 (0.0002) a | 0.0006 (0.0002) ab | 0.0004 (0.0002) b |
| Na | 0.0091 (0.0051) a | 0.012 (0.002) a | 0.0086 (0.0046) a | 0.0069 (0.0028) a |
| Ni | 0.0003 (0) a | 0.0008 (0.0004) a | 0.0003 (0.0001) a | 0.0003 (0.0001) a |
| Zn | 0.0012 (0.0001) ab | 0.0013 (0.0007) a | 0.0012 (0.0005) ab | 0.0006 (0.0003) b |
| Nutrient (kg ha−1) | Site | |||
|---|---|---|---|---|
| SAN | ZAM | DOS | VIL | |
| NO3−-N | 4.48 (4.17) ab | 4.69 (4.34) ab | 8.13 (7.97) a | 1.61 (0.79) b |
| NH4+-N | 51.67 (9.78) a | 44.31 (18.3) ab | 37.54 (6.28) ab | 27.14 (10.73) b |
| PO4-P | 3.85 (0.65) a | 27.91 (35.47) a | 11.38 (10.8) a | 11.08 (8.08) a |
| Na | 14.33 (2.94) ab | 22.25 (8.61) a | 5.42 (1) c | 10.62 (2.37) b |
| K | 851.14 (83.72) a | 192.44 (88.3) b | 96.34 (12.23) c | 166.47 (40.42) b |
| Ca | 2245.51 (2460.26) a | 114.79 (33.69) b | 49.17 (14.07) c | 91.42 (26.99) b |
| Mg | 605.14 (738.28) a | 9.51 (8.8) b | 13.18 (3.5) ab | 18.19 (16.75) ab |
| Al | 5088.61 (1216.28) a | 2964.51 (1472.39) a | 1342.89 (615.01) b | 1577.44 (852.61) b |
| Fe | 79.95 (46.57) a | 65.66 (39.46) a | 93.38 (57.46) a | 39.75 (10.41) a |
| Mn | 17 (25.2) a | nd | 4.73 (7.02) a | nd |
| Forest Attribute | Site | |||
|---|---|---|---|---|
| SAN | ZAM | DOS | VIL | |
| Stand age (years) | 20 | 18 | 22 | 14 |
| Stand Density (SD) (trees ha−1) | 763.9 (0) b | 1684 (797.9) a | 729.2 (128.7) b | 954.9 (77.2) a |
| Basal area (BA) (m2 ha−1) | 44.2 (2.7) a | 47.3 (6.2) a | 25.5 (3.1) b | 17.1 (1.7) c |
| Diameter at breast height (DBH) (cm) | 27.4 (0.9) a | 19 (7.1) b | 20.4 (0.3) ab | 14.6 (1.5) c |
| Quadratic mean diameter (QMD) (cm) | 27.7 (1.1) a | 19.9 (7.4) b | 21.3 (0.5) ab | 15.3 (1.4) c |
| Stand height (SH) (m) | 23.7 (1.3) a | 17.8 (4.9) b | 18 (1.8) b | 10.3 (0.5) c |
| Dominant height (DH) (m) | 22.3 (1.8) a | 15 (1.7) b | 15.9 (1.2) b | 9 (0.4) c |
| Stand Volume (m3 ha−1) | 501.6 (71.7) a | 401.2 (61.1) a | 203.8 (23) b | 75.2 (11.7) c |
| DH20 (m) | 22.3 (1.8) a | 16.6 (1.9) a | 14.5 (1.01) b | 12.9 (0.5) b |
| Environmental Variables | Forest Site Class | ||
|---|---|---|---|
| A (DH20 = 22.3 m) | B (DH20 = 16.6 m) | C (DH20 = 13.4 m) | |
| Temperature (TEM) (°C ) | 9 (0) b | 12 (0) a | 10 (1.48) b |
| Potential Evapotranspiration (PET) (mm y−1) | 1038 (0) c | 1050 (0) a | 1040 (2.97) b |
| Aspect (decimal degrees) | 360 (0) a | 315 (0) a | 315 (0) a |
| Slope (%) | 30 (1.48) b | 41 (5.93) ab | 42 (4.45) a |
| Altitude (m a.s.l.) | 2430 (13.34) a | 2234 (16.31) c | 2393 (48.93) b |
| Maximum root depth (MRD) (cm) | 170 (14.83) a | 100 (29.65) b | 150 (44.48) a |
| Dominant root depth (DRD) (cm) | 30 (0) a | 50 (0) a | 40 (14.83) a |
| Forest floor thickness (FFT) (cm) | 3.6 (0.59) c | 12 (5.29) a | 7 (1.78) b |
| Stoniness (%) | 18.38 (15.63) a | 15.51 (9.74) a | 28.76 (25.39) a |
| Bulk density (Mg m−3) | 1.13 (0.1) b | 1.33 (0.16) a | 1.09 (0.17) b |
| Clay (%) | 50.85 (5.13) a | 19.45 (1.19) b | 23.3 (7.41) b |
| Silt (%) | 20.85 (3.11) c | 45.35 (2.04) a | 35.7 (10.23) b |
| Sand (%) | 24.7 (1.85) b | 35.65 (0.74) ab | 39 (7.41) a |
| pH in water | 5.41 (0) a | 4.39 (0.36) b | 4.64 (0.24) b |
| Base saturation (%) | 68.48 (2.13) a | 27.49 (12.85) b | 33.95 (11.95) b |
| Short to medium term available nutrient stocks (Mg ha−1) | |||
| N | 0.41 (0.01) b | 0.76 (0.25) a | 0.51 (0.23) b |
| P | 0.03 (0) b | 0.07 (0.04) a | 0.03 (0.01) b |
| K | 0.93 (0.03) a | 0.33 (0.21) ab | 0.22 (0.05) b |
| Ca | 2.48 (2.48) a | 0.18 (0.03) b | 0.14 (0.09) b |
| Mg | 0.65 (0.76) a | 0.04 (0.02) b | 0.05 (0.02) b |
| Total Exchange nutrient stocks (Mg ha−1) | 9.05 (4.53) a | 3.46 (1.73) b | 2.01 (0.85) c |
| Nutrient ratios | |||
| N/P | 4.61 (0.13) a | 5.95 (0.35) a | 4.01 (2.11) a |
| N/K | 0.21 (0.11) a | 0.1 (0.03) a | 0.1 (0.07) a |
| N/Ca | 2.02 (1.67) a | 2.28 (2.94) a | 6.81 (7.29) a |
| available/total stocks | 1.17 (0.5) a | 0.65 (0.25) a | 0.34 (0.19) b |
| Ca/Mg | 3.26 (0.47) ab | 4.87 (2.33) a | 2.25 (1.24) b |
| C/N | 13.86 (1.11) b | 23.18 (5.44) ab | 22.45 (3.22) a |
| K * | 0.08 (0.04) b | 0.41 (0.16) a | 0.34 (0.17) a |
| Nutrient Stocks | VIP | Spearman Correlation with DH20 | |
|---|---|---|---|
| DH20 Cor | p-Value | ||
| Ca | 0.04 | 0.58 | 0.004 |
| K | 0.08 | 0.52 | 0.012 |
| Mg | 0.04 | 0.30 | 0.159 |
| N | 0.07 | 0.22 | 0.317 |
| P | 0.00 | 0.28 | 0.195 |
| Forest Site Classes | Description |
|---|---|
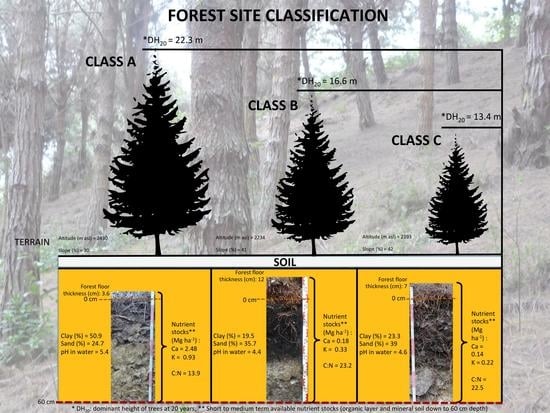
| Class A: “High forest productivity” (dominant height of pine trees in 20 year old plantations approximately 22 m). | Sites with the highest soil fertility: short to medium term available nutrient stocks are highest with total exchangeable nutrient stocks of approximately 9 Mg ha−1; particularly, Ca and K (2.5 and 0.9 Mg ha−1, respectively). This is associated with two times higher clay contents (51%) and more than 10 fold lower concentration of H3O+. Stocks of short and medium term available macronutrients (N and P) are lower or similar to the other forest site classes. This together with the narrowest C:N ratio and the lowest forest floor thickness hints towards efficient nutrient cycling processes. In the study region this forest site class occurs in the highest altitude (around 2430 m a.s.l.) at slopes with the lowest steepness (30%). |
| Class B: “Middle forest productivity” (dominant height of pine trees in 20 year old plantations approximately 17 m). | Sites with middle soil fertility: the total exchangeable nutrient stocks are 2.6 times lower than in the forest site class of high productivity. The short to medium term available stocks of Ca and K show a notable decrease in comparison to the high forest site class (0.18 and 0.33 Mg ha−1, respectively). In contrast, stocks of short and medium term available N and P as well as forest floor thickness are highest. The concentration of H3O+ is by tendency even higher than in the low forest productivity class. In the study region this site class is located at the lowest altitude in areas with slopes around 41%. |
| Class C: “Low forest productivity” (dominant height of pine trees in 20 year old plantations approximately 13 m). | Site with low soil fertility: the stocks of total available nutrients are approximately 4.5 times lower than in the forest site class of high productivity but 1.7 times lower than at sites of middle productivity. The short to medium term available stocks of Ca, K are the lowest (0.14 and 0.22 Mg ha−1, respectively) whereas N and P stocks are slightly higher or similar to those in the high forest productivity class. The content of sand is highest in the soils with low forest productivity (39%). In the study region this site class occurs in areas with middle altitude and with the steepness similar to the sites of middle productivity (slope 42%). |
| Rating | Wolff and Riek [50] | Forest Site Classes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value Range (Mg ha−1) | A (DH20 = 22.3 m) | B (DH20 = 16.6 m) | C (DH20 = 13.4 m) | |||||||||
| K | Ca | Mg | K | Ca | Mg | K | Ca | Mg | K | Ca | Mg | |
| Very low | <0.2 | <0.2 | <0.05 | 0.18 | 0.04 | 0.14 | ||||||
| Low | 0.2–0.4 | 0.2–0.4 | 0.05–0.1 | 0.33 | 0.22 | 0.05 | ||||||
| Moderate | 0.4–0.6 | 0.4–0.8 | 0.1–0.2 | |||||||||
| Medium | 0.6–0.8 | 0.8–2.0 | 0.2–0.5 | |||||||||
| Moderately high | 0.8–1.2 | 2.0–4.0 | 0.5–1.0 | 0.93 | 2.48 | 0.65 | ||||||
| High | 1.2–1.6 | 4.0–8.0 | 1.0–2.0 | |||||||||
| Very high | ≥1.6 | ≥8.0 | ≥2.0 | |||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quichimbo, P.; Jiménez, L.; Veintimilla, D.; Tischer, A.; Günter, S.; Mosandl, R.; Hamer, U. Forest Site Classification in the Southern Andean Region of Ecuador: A Case Study of Pine Plantations to Collect a Base of Soil Attributes. Forests 2017, 8, 473. https://doi.org/10.3390/f8120473
Quichimbo P, Jiménez L, Veintimilla D, Tischer A, Günter S, Mosandl R, Hamer U. Forest Site Classification in the Southern Andean Region of Ecuador: A Case Study of Pine Plantations to Collect a Base of Soil Attributes. Forests. 2017; 8(12):473. https://doi.org/10.3390/f8120473
Chicago/Turabian StyleQuichimbo, Pablo, Leticia Jiménez, Darío Veintimilla, Alexander Tischer, Sven Günter, Reinhard Mosandl, and Ute Hamer. 2017. "Forest Site Classification in the Southern Andean Region of Ecuador: A Case Study of Pine Plantations to Collect a Base of Soil Attributes" Forests 8, no. 12: 473. https://doi.org/10.3390/f8120473
APA StyleQuichimbo, P., Jiménez, L., Veintimilla, D., Tischer, A., Günter, S., Mosandl, R., & Hamer, U. (2017). Forest Site Classification in the Southern Andean Region of Ecuador: A Case Study of Pine Plantations to Collect a Base of Soil Attributes. Forests, 8(12), 473. https://doi.org/10.3390/f8120473