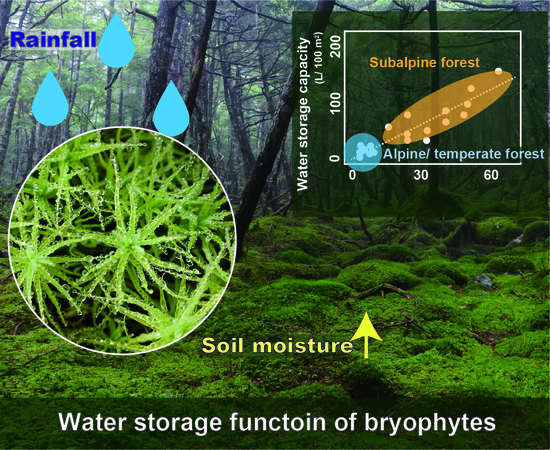
Evaluation of the Water-Storage Capacity of Bryophytes along an Altitudinal Gradient from Temperate Forests to the Alpine Zone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling and Water Storage Capacity
2.3. Water Storage Capacity at Quadrat, Substrate, and Plot Scales
2.4. Modeling
3. Results
3.1. Water Storage Capacity of Bryophyte Communities at the Quadrat Scale
3.1.1. Comparison of Water Storage Capacity of Bryophyte Communities
3.1.2. Water Storage Capacity of Bryophytes and Soil Moisture
3.1.3. Altitudinal Patterns of Water Storage Capacity of Bryophyte Communities at the Quadrat Scale
3.2. Water Storage Capacity of Bryophyte Communities on Each Substrate
3.3. Water Storage Capacity of Bryophyte Communities per Plot
4. Discussion
4.1. Water Storage Capacity at the Quadrat Scale
4.2. Influence of Forest Floor Bryophytes on Below Ground Processes
4.3. Water Storage Capacity at the Substrate/Plot Scale
4.4. Changes to Water Storage Capacity by Climatic Change
5. Conclusions
Funding
Conflicts of Interest
References
- Vose, J.M.; Sun, G.; Ford, C.R.; Bredemeier, M.; Otsuki, K.; Wei, X.; Zhang, Z.; Zhang, L. Forest ecohydrological research in the 21st century: What are the critical needs? Ecohydrology 2011, 4, 146–158. [Google Scholar] [CrossRef]
- Brown, A.E.; Zhang, L.; McMahon, T.A.; Western, A.W.; Vertessy, R.A. A review of paired catchment studies for determining changes in water yield resulting from alterations in vegetation. J. Hydrol. 2005, 310, 28–61. [Google Scholar] [CrossRef]
- Farley, K.A.; Jobbagy, E.G.; Jackson, R.B. Effects of afforestation on water yield: A global synthesis with implications for policy. Glob. Chang. Biol. 2005, 11, 1565–1576. [Google Scholar] [CrossRef]
- Postel, S.L.; Thompson, B.H. Watershed protection: Capturing the benefits of nature’s water supply services. Nat. Resour. Forum 2005, 29, 98–108. [Google Scholar] [CrossRef]
- Guo, Z.; Xiao, X.; Gan, Y.; Zheng, Y. Ecosystem functions, services and their values—A case study in Xingshan County of China. Ecol. Econ. 2001, 38, 141–154. [Google Scholar] [CrossRef]
- Mashayekhi, Z.; Panahi, M.; Karami, M.; Khalighi, S.; Malekian, A. Economic valuation of water storage function of forest ecosystems (case study: Zagros Forests, Iran). J. For. Res. 2010, 21, 293–300. [Google Scholar] [CrossRef]
- Ataroff, M.; Rada, F. Deforestation impact on water dynamics in a Venezuelan Andean cloud forest. AMBIO 2000, 29, 440–444. [Google Scholar] [CrossRef]
- Keim, R.F.; Skaugset, A.E.; Weiler, M. Storage of water on vegetation under simulated rainfall of varying intensity. Adv. Water Resour. 2006, 29, 974–986. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Zagt, R.; Van Leerdam, A.; Van Ek, R.; Broekhoven, A.; Van Genderen, M. Hydrological properties of the epiphyte mass of a montane tropical rain forest, colombia. Vegetatio 1990, 89, 183–192. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Bai, Z.; Lv, C. Effects of vegetation on runoff and soil erosion on reclaimed land in an opencast coal-mine dump in a loess area. Catena 2015, 128, 44–53. [Google Scholar] [CrossRef]
- Le Bissonnais, Y.; Lecomte, V.; Cerdan, O. Grass strip effects on runoff and soil loss. Agronomie 2004, 24, 129–136. [Google Scholar] [CrossRef]
- Chamizo, S.; Cantón, Y.; Rodríguez-Caballero, E.; Domingo, F.; Escudero, A. Runoff at contrasting scales in a semiarid ecosystem: A complex balance between biological soil crust features and rainfall characteristics. J. Hydrol. 2012, 452–453, 130–138. [Google Scholar] [CrossRef]
- Belnap, J. The potential roles of biological soil crusts in dryland hydrologic cycles. Hydrol. Process. 2006, 20, 3159–3178. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Creed, I.F.; Spargo, A.T.; Jones, J.A.; Buttle, J.M.; Adams, M.B.; Beall, F.D.; Booth, E.G.; Campbell, J.L.; Clow, D.; Elder, K.; et al. Changing forest water yields in response to climate warming: Results from long-term experimental watershed sites across North America. Glob. Chang. Biol. 2014, 20, 3191–3208. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Neilson, R.P.; Lenihan, J.M.; Drapek, R.J. Global patterns in the vulnerability of ecosystems to vegetation shifts due to climate change. Glob. Ecol. Biogeogr. 2010, 19, 755–768. [Google Scholar] [CrossRef]
- Proctor, M.C.; Tuba, Z. Poikilohydry and homoihydry: Antithesis or spectrum of possibilities? New Phytol. 2002, 156, 327–349. [Google Scholar] [CrossRef]
- He, X.; He, K.S.; Hyvönen, J. Will bryophytes survive in a warming world? Perspect. Plant. Ecol. Evol. Syst. 2016, 19, 49–60. [Google Scholar] [CrossRef]
- Oishi, Y. Urban heat island effects on moss gardens in Kyoto, Japan. Lands. Ecol. Eng. 2018. [Google Scholar] [CrossRef]
- Pypker, T.G.; Unsworth, M.H.; Bond, B.J. The role of epiphytes in rainfall interception by forests in the Pacific Northwest. I. Laboratory measurements of water storage. Can. J. For. Res. 2006, 36, 809–818. [Google Scholar] [CrossRef]
- Pócs, T. The epiphytic biomass and its effect on the water balance of two rain forest types in the Uluguru Mountains (Tanzania, East Africa). Acta Bot. Acad. Sci. Hung. 1980, 26, 143–167. [Google Scholar]
- Hölscher, D.; Köhler, L.; van Dijk, A.I.; Bruijnzeel, L.S. The importance of epiphytes to total rainfall interception by a tropical montane rain forest in Costa Rica. J. Hydrol. 2004, 292, 308–322. [Google Scholar] [CrossRef]
- Ah-Peng, C.; Cardoso, A.W.; Flores, O.; West, A.; Wilding, N.; Strasberg, D.; Hedderson, T.A.J. The role of epiphytic bryophytes in interception, storage, and the regulated release of atmospheric moisture in a tropical montane cloud forest. J. Hydrol. 2017, 548, 665–673. [Google Scholar] [CrossRef]
- Chang, S.-C.; Lai, I.-L.; Wu, J.-T. Estimation of fog deposition on epiphytic bryophytes in a subtropical montane forest ecosystem in Northeastern Taiwan. Atmos. Res. 2002, 64, 159–167. [Google Scholar] [CrossRef]
- Köhler, L.; Tobón, C.; Frumau, K.A.; Bruijnzeel, L.S. Biomass and water storage dynamics of epiphytes in old-growth and secondary montane cloud forest stands in Costa Rica. Plant. Ecol. 2007, 193, 171–184. [Google Scholar] [CrossRef]
- Michel, P.; Payton, I.J.; Lee, W.G.; During, H.J. Impact of disturbance on above-ground water storage capacity of bryophytes in New Zealand indigenous tussock grassland ecosystems. N. Z. J. Ecol. 2013, 114–126. [Google Scholar]
- Elumeeva, T.G.; Soudzilovskaia, N.A.; During, H.J.; Cornelissen, J.H. The importance of colony structure versus shoot morphology for the water balance of 22 subarctic bryophyte species. J. Veg. Sci. 2011, 22, 152–164. [Google Scholar] [CrossRef]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Zimov, S.A.; Chuprynin, V.; Oreshko, A.; Chapin III, F.; Reynolds, J.; Chapin, M. Steppe-tundra transition: A herbivore-driven biome shift at the end of the Pleistocene. Am. Nat. 1995, 146, 765–794. [Google Scholar] [CrossRef]
- Hobbie, S.E.; Schimel, J.P.; Trumbore, S.E.; Randerson, J.R. Controls over carbon storage and turnover in high-latitude soils. Glob. Chang. Biol. 2000, 6, 196–210. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.C.; Iwatsuki, Z. Hot spots of mosses in East Asia. Anal. Inst. Biol. Ser. Bot. 1996, 67, 159–167. [Google Scholar]
- Geffert, J.L.; Frahm, J.-P.; Barthlott, W.; Mutke, J. Global moss diversity: Spatial and taxonomic patterns of species richness. J. Bryol. 2013, 35, 1–11. [Google Scholar] [CrossRef]
- Sun, S.-Q.; Wu, Y.-H.; Wang, G.-X.; Zhou, J.; Yu, D.; Bing, H.-J.; Luo, J. Bryophyte species richness and composition along an altitudinal gradient in Gongga Mountain, China. PLoS ONE 2013, 8, e58131. [Google Scholar] [CrossRef] [PubMed]
- Grau, O.; Grytnes, J.A.; Birks, H. A comparison of altitudinal species richness patterns of bryophytes with other plant groups in Nepal, Central Himalaya. J. Biogeogr. 2007, 34, 1907–1915. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Past Meteorological Data (Nobeyama). Available online: http://www.data.jma.go.jp/obd/stats/etrn/view/nml_amd_ym.php?prec_no=48&block_no=0415&year=&month=&day=&view= (accessed on 12 April 2018).
- Japan Meteorological Agency. Top Ten in Recorded History. Available online: http://www.data.jma.go.jp/obd/stats/etrn/view/rank_a.php?prec_no=48&block_no=0415&year=&month=&day=&view= (accessed on 12 April 2018).
- Oishi, Y. Comparison of moss and pine needles as bioindicators of transboundary polycyclic aromatic hydrocarbon pollution in central japan. Environ. Pollut. 2018, 234, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Bates, J. Is life-form a useful concept in bryophyte ecology? Oikos 1998, 223–237. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 12 April 2018).
- Proctor, M.C. The bryophyte paradox: Tolerance of desiccation, evasion of drought. Plant. Ecol. 2000, 151, 41–49. [Google Scholar] [CrossRef]
- Michel, P.; Lee, W.G.; During, H.J.; Cornelissen, J.H.C. Species traits and their non-additive interactions control the water economy of bryophyte cushions. J. Ecol. 2012, 100, 222–231. [Google Scholar] [CrossRef]
- Maestre, F.T.; Huesca, M.; Zaady, E.; Bautista, S.; Cortina, J. Infiltration, penetration resistance and microphytic crust composition in contrasted microsites within a Mediterranean semi-arid steppe. Soil Biol. Biochem. 2002, 34, 895–898. [Google Scholar] [CrossRef]
- Eldridge, D.; Rosentreter, R. Morphological groups: A framework for monitoring microphytic crusts in arid landscapes. J. Arid Environ. 1999, 41, 11–25. [Google Scholar] [CrossRef]
- Nakatsubo, T. The role of bryophytes in terrestrial ecosystems with special reference to forests and volcanic deserts. Jpn. J. Ecol. 1997, 47, 43–54. [Google Scholar] [CrossRef]
- LaMalfa, E.M.; Ryle, R. Differential snowpack accumulation and water dynamics in aspen and conifer communities: Implications for water yield and ecosystem function. Ecosystems 2008, 11, 569–581. [Google Scholar] [CrossRef]
- Beringer, J.; Lynch, A.H.; Chapin, F.S., III; Mack, M.; Bonan, G.B. The representation of arctic soils in the land surface model: The importance of mosses. J. Clim. 2001, 14, 3324–3335. [Google Scholar] [CrossRef]
- Kalina, M.F.; Stopper, S.; Zambo, E.; Puxbaum, H. Altitude-dependent wet, dry and occult nitrogen deposition in an alpine region. Environ. Sci. Pollut. Res. 2002, 9, 16–22. [Google Scholar] [CrossRef]
- Suding, K.N.; Lavorel, S.; Chapin, F.S.; Cornelissen, J.H.C.; DÍAz, S.; Garnier, E.; Goldberg, D.; Hooper, D.U.; Jackson, S.T.; Navas, M.-L. Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Chang. Biol. 2008, 14, 1125–1140. [Google Scholar] [CrossRef]
- Aiguo, D. Drought under global warming: A review. WIREs Clim. Chang. 2011, 2, 45–65. [Google Scholar] [CrossRef]
- Robinson, C.; Wookey, P.; Parsons, A.; Potter, J.; Callaghan, T.; Lee, J.; Press, M.; Welker, J. Responses of plant litter decomposition and nitrogen mineralisation to simulated environmental change in a high arctic polar semi-desert and a subarctic dwarf shrub heath. Oikos 1995, 503–512. [Google Scholar] [CrossRef]
- Fisk, M.C.; Schmidt, S.K.; Seastedt, T.R. Topographic patterns of above-and belowground production and nitrogen cycling in alpine tundra. Ecology 1998, 79, 2253–2266. [Google Scholar] [CrossRef]




| Altitude (m) | Trail 1 | Bryophyte Community (Life form Type) | n | FW (g/100 cm2) | DW (g/100 cm2) | WSCf-q (g/100 cm2) | WSCd-q (g/100 cm2) | Cover (m2/plot) |
|---|---|---|---|---|---|---|---|---|
| Soil | ||||||||
| 1800 | E | Dic nipn–Pog con (T–T: T) | 3 | 8.05 ± 1.37 | 2.80 ± 0.39 | 13.79 ± 4.04 | 19.04 ± 4.17 | 5 |
| 2000 | E | Hyl spl–Ple shr (W–W: W) | 3 | 9.28 ± 2.97 | 3.54 ± 0.74 | 20.27 ± 5.86 | 26.03 ± 8.08 | 7 |
| 2200 | E | Pog con–Hyl spl (T–W) | 3 | 17.12 ± 1.07 | 5.28 ± 0.96 | 14.11 ± 4.08 | 25.95 ± 3.93 | 24 |
| 2400 | E | Pog con (T) | 3 | 9.71 ± 1.47 | 3.38 ± 0.47 | 10.18 ± 1.96 | 16.51 ± 2.47 | 32 |
| 2600 | E | Pog jap (T) | 3 | 38.96 ± 9.52 | 14.81 ± 3.65 | 32.89 ± 3.94 | 57.03 ± 7.08 | 24 |
| 2800 | E | Dic maj–Het aff–Ple shr (T–Sm–W) | 3 | 7.21 ± 4.47 | 2.53 ± 1.47 | 12.11 ± 7.95 | 16.79 ± 10.32 | 10 |
| 1800 | W | Hyl spl–Ple shr (W–W: W) | 3 | 15.90 ± 5.37 | 5.23 ± 0.19 | 24.78 ± 5.40 | 35.47 ± 0.20 | 63 |
| 2000 | W | Hyl spl (W) | 3 | 10.03 ± 0.96 | 4.57 ± 0.52 | 24.39 ± 6.44 | 30.18 ± 6.90 | 51 |
| 2200 | W | Pog jap–Hyl spl–Ple shr (T–W–W: T–W) | 3 | 18.83 ± 6.51 | 5.56 ± 1.75 | 16.45 ± 9.42 | 29.73 ± 8.59 | 40 |
| 2400 | W | Pog jap–Dic maj–Ple shr (T–T–W: T–W) | 3 | 11.69 ± 6.43 | 3.90 ± 1.83 | 16.86 ± 10.54 | 24.65 ± 14.56 | 48 |
| 2600 | W | Dic maj–Hyl spl (T–W) | 3 | 15.12 ± 3.90 | 4.53 ± 1.16 | 19.79 ± 3.75 | 30.38 ± 6.21 | 50 |
| 2800 | W | Cor fas (Cu) | 3 | 22.24 ± 3.00 | 7.34 ± 1.85 | 31.02 ± 11.71 | 45.92 ± 7.03 | 5 |
| Logs | ||||||||
| 2000 | E | Hyl spl–Ple shr (W–W: W) | 3 | 6.20 ± 0.73 | 2.91 ± 0.51 | 12.21 ± 5.60 | 15.49 ± 5.59 | 3 |
| 2200 | E | Het aff–Ple shr (Sm–W) | 3 | 14.85 ± 3.33 | 4.87 ± 0.47 | 25.51 ± 2.24 | 35.49 ± 1.78 | 32 |
| 2400 | E | Now cur–Ple shr (Tl–W) | 3 | 5.45 ± 2.16 | 2.00 ± 1.37 | 10.71 ± 7.20 | 14.16 ± 8.00 | 8 |
| 2600 | E | Rig rob–Ple shr (Rm–W) | 3 | 13.35 ± 2.62 | 6.52 ± 0.08 | 35.47 ± 9.30 | 42.30 ± 8.77 | 4 |
| 1800 | W | Hyp pli–Hyl spl–Ple shr (Sm–W–W: Sm–W) | 4 | 12.87 ± 3.75 | 5.29 ± 1.05 | 29.20 ± 10.44 | 36.78 ± 12.15 | 8 |
| 2000 | W | Het aff–Hyl spl (W–W: W) | 4 | 9.05 ± 1.87 | 3.24 ± 0.69 | 18.59 ± 8.12 | 24.40 ± 5.64 | 24 |
| 2200 | W | Hyl spl–Ple shr (W–W: W) | 3 | 17.40 ± 2.88 | 5.38 ± 1.67 | 21.66 ± 11.15 | 33.68 ± 7.42 | 40 |
| 2400 | W | Hyl spl–Ple shr (W–W: W) | 3 | 13.84 ± 3.90 | 6.32 ± 1.20 | 36.00 ± 16.55 | 43.52 ± 17.07 | 16 |
| Life Form | T | Cu | W | Rm–W | Sm–W | T–W | Tl–W | T–Sm–W |
|---|---|---|---|---|---|---|---|---|
| n | 9 | 3 | 21 | 3 | 8 | 12 | 3 | 3 |
| WSCf-q (g/100 cm2) | ||||||||
| Average | 18.95 | 31.02 | 23.55 | 35.47 | 23.89 | 16.80 | 10.71 | 12.11 |
| SD | 10.99 | 11.71 | 9.98 | 9.30 | 9.45 | 6.8 | 7.20 | 7.95 |
| Significance | ab | ab | ab | b | ab | ab | a | ab |
| WSCd-q (g/100 cm2) | ||||||||
| Average | 30.86 | 45.92 | 31.40 | 42.30 | 30.59 | 27.68 | 14.16 | 16.79 |
| SD | 20.12 | 7.03 | 10.94 | 8.77 | 10.34 | 8.26 | 8.01 | 10.32 |
| Significance | ab | b | ab | ab | ab | ab | a | ab |
| Altitude (m) | E Trail | W Trail | ||
|---|---|---|---|---|
| WSCf-Plot (L/100 m2) | WSCd-Plot (L/100 m2) | WSCf-Plot (L/100 m2) | WSCd-Plot (L/100 m2) | |
| 1800 | 6.895 | 9.522 | 179.453 | 252.866 |
| 2000 | 17.853 | 22.850 | 168.982 | 212.466 |
| 2200 | 115.507 | 133.275 | 152.469 | 253.600 |
| 2400 | 40.459 | 64.939 | 138.523 | 187.947 |
| 2600 | 93.133 | 153.808 | 98.967 | 151.917 |
| 2800 | 12.107 | 16.790 | 15.508 | 22.958 |
| Average ± SD | 47.77 ± 41.89 | 66.73 ± 57.31 | 125.65 ± 55.53 | 180.29 ± 78.84 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oishi, Y. Evaluation of the Water-Storage Capacity of Bryophytes along an Altitudinal Gradient from Temperate Forests to the Alpine Zone. Forests 2018, 9, 433. https://doi.org/10.3390/f9070433
Oishi Y. Evaluation of the Water-Storage Capacity of Bryophytes along an Altitudinal Gradient from Temperate Forests to the Alpine Zone. Forests. 2018; 9(7):433. https://doi.org/10.3390/f9070433
Chicago/Turabian StyleOishi, Yoshitaka. 2018. "Evaluation of the Water-Storage Capacity of Bryophytes along an Altitudinal Gradient from Temperate Forests to the Alpine Zone" Forests 9, no. 7: 433. https://doi.org/10.3390/f9070433
APA StyleOishi, Y. (2018). Evaluation of the Water-Storage Capacity of Bryophytes along an Altitudinal Gradient from Temperate Forests to the Alpine Zone. Forests, 9(7), 433. https://doi.org/10.3390/f9070433