Why Are Algal Viruses Not Always Successful?
Abstract
:1. Introduction
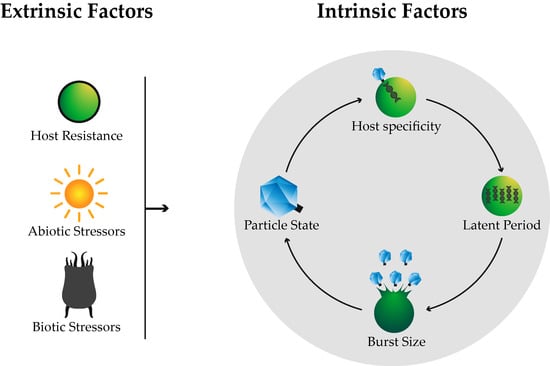
2. Intrinsic Factors
2.1. Host Specificity
2.2. Life Cycle
2.2.1. Latent Period
2.2.2. Burst Size
3. Extrinsic Factors
3.1. Host Resistance
3.2. Biotic Stressors
3.2.1. Predation
3.2.2. Virophages
3.2.3. Indirect Interactions
3.3. Abiotic Stressors
3.3.1. Temperature
3.3.2. Sunlight and UV Radiation
3.3.3. Nutrient Concentrations
3.3.4. Non-Host Particles
3.3.5. CO2 Concentrations/pH
3.3.6. Salinity
4. Virus Evolution
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spencer, R. A Marine Bacteriophage. Nature 1955, 175, 690–691. [Google Scholar] [CrossRef] [PubMed]
- Safferman, R.S.; Morris, M.E. Algal Virus: Isolation. Science 1963, 140, 679–680. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.; Skotnicki, A.H.; Gardiner, J.E.; Walker, E.S.; Hollings, M. A tobamovirus of a green alga. Virology 1975, 64, 571–574. [Google Scholar] [CrossRef]
- Bergh, O.; Børsheim, K.Y.; Bratbak, G.; Heldal, M. High abundance of viruses found in aquatic environments. Nature 1989, 340, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Torrella, F.; Morita, R.Y. Evidence by electron micrographs for a high incidence of bacteriophage particles in the waters of Yaquina Bay, oregon: Ecological and taxonomical implications. Appl. Environ. Microbiol. 1979, 37, 774–778. [Google Scholar] [PubMed]
- Jacquet, S.; Miki, T.; Noble, R.; Peduzzi, P.; Wilhelm, S. Viruses in aquatic ecosystems: Important advancements of the last 20 years and prospects for the future in the field of microbial oceanography and limnology. Adv. Oceanogr. Limnol. 2010, 1, 97–141. [Google Scholar] [CrossRef]
- Danovaro, R.; Corinaldesi, C.; Filippini, M.; Fischer, U.R.; Gessner, M.O.; Jacquet, S.; Magagnini, M.; Velimirov, B. Viriobenthos in freshwater and marine sediments: A review. Freshw. Biol. 2008, 53, 1186–1213. [Google Scholar] [CrossRef]
- Wigington, C.H.; Sonderegger, D.; Brussaard, C.P.D.; Buchan, A.; Finke, J.F.; Fuhrman, J.A.; Lennon, J.T.; Middelboe, M.; Suttle, C.A.; Stock, C.; et al. Re-examination of the relationship between marine virus and microbial cell abundances. Nat. Microbiol. 2016, 1, 15024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breitbart, M.; Bonnain, C.; Malki, K.; Sawaya, N.A. Phage puppet masters of the marine microbial realm. Nat. Microbiol. 2018, 3, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, M.; Salamon, P.; Andresen, B.; Mahaffy, J.M.; Segall, A.M.; Mead, D.; Azam, F.; Rohwer, F. Genomic analysis of uncultured marine viral communities. Proc. Natl. Acad. Sci. USA 2002, 99, 14250–14255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hingamp, P.; Grimsley, N.; Acinas, S.G.; Clerissi, C.; Subirana, L.; Poulain, J.; Ferrera, I.; Sarmento, H.; Villar, E.; Lima-Mendez, G.; et al. Exploring nucleo-cytoplasmic large DNA viruses in Tara Oceans microbial metagenomes. ISME J. 2013, 7, 1678–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, A.; Poulain, J.; Engelen, S.; Labadie, K.; Romac, S.; Ferrera, I.; Albini, G.; Aury, J.-M.; Belser, C.; Bertrand, A.; et al. Viral to metazoan marine plankton nucleotide sequences from the Tara Oceans expedition. Sci. Data 2017, 4, 170093. [Google Scholar] [CrossRef] [PubMed]
- Paez-Espino, D.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Thomas, A.D.; Huntemann, M.; Mikhailova, N.; Rubin, E.; Ivanova, N.N.; Kyrpides, N.C. Uncovering Earth’s virome. Nature 2016, 536, 425–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brum, J.R.; Schenck, R.O.; Sullivan, M.B. Global morphological analysis of marine viruses shows minimal regional variation and dominance of non-tailed viruses. ISME J. 2013, 7, 1738–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brum, J.R.; Ignacio-Espinoza, J.C.; Roux, S.; Doulcier, G.; Acinas, S.G.; Alberti, A.; Chaffron, S.; Cruaud, C.; de Vargas, C.; Gasol, J.M.; Gorsky, G.; et al. Patterns and ecological drivers of ocean viral communities. Science 2015, 348, 1261498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, S.; Brum, J.R.; Dutilh, B.E.; Sunagawa, S.; Duhaime, M.B.; Loy, A.; Poulos, B.T.; Solonenko, N.; Lara, E.; Poulain, J.; et al. Ecogenomics and potential biogeochemical impacts of globally abundant ocean viruses. Nature 2016, 537, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Short, S.M.; Suttle, C.A. Sequence Analysis of Marine Virus Communities Reveals that Groups of Related Algal Viruses Are Widely Distributed in Nature. Appl. Environ. Microbiol. 2002, 68, 1290–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suttle, C.A. Marine viruses—Major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Short, S.M. The ecology of viruses that infect eukaryotic algae. Environ. Microbiol. 2012, 14, 2253–2271. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, M. Marine Viruses: Truth or Dare. Ann. Rev. Mar. Sci. 2012, 4, 425–448. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, M.A. The ecological virus. Stud. Hist. Philos. Sci. Part C Stud. Hist. Philos. Biol. Biomed. Sci. 2016, 59, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, C.P.D.; Baudoux, A.C.; Rodríguez-Valera, F. Marine Viruses. In The Marine Microbiome; Springer: Cham, Switzerland, 2016; pp. 155–183. [Google Scholar]
- Middelboe, M.; Brussaard, C. Marine Viruses: Key Players in Marine Ecosystems. Viruses 2017, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, M.; Thompson, L.R.; Suttle, C.A.; Sullivan, M.B. Exploring the Vast Diversity of Marine Viruses. Oceanography 2007, 20, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Fuhrman, J.A. Marine viruses and their biogeochemical and ecological effects. Nature 1999, 399, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Weitz, J.; Wilhelm, S. Ocean viruses and their effects on microbial communities and biogeochemical cycles. F1000 Biol. Rep. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A.; Chan, A.M.; Cottrell, M.T. Infection of phytoplankton by viruses and reduction of primary productivity. Nature 1990, 347, 467–469. [Google Scholar] [CrossRef]
- Waters, R.E.; Chan, A.T. Micromonas pusilla Virus: The Virus Growth Cycle and Associated Physiological Events within the Host Cells; Host Range Mutation. J. Gen. Virol. 1982, 63, 199–206. [Google Scholar] [CrossRef]
- Van Etten, J.L.; Burbank, D.E.; Xia, Y.; Meints, R.H. Growth cycle of a virus, PBCV-1, that infects Chlorella-like algae. Virology 1983, 126, 117–125. [Google Scholar] [CrossRef]
- Nagasaki, K.; Tomaru, Y.; Takao, Y.; Nishida, K.; Shirai, Y.; Suzuki, H.; Nagumo, T. Previously Unknown Virus Infects Marine Diatom. Appl. Environ. Microbiol. 2005, 71, 3528–3535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomaru, Y.; Shirai, Y.; Suzuki, H.; Nagumo, T.; Nagasaki, K. Isolation and characterization of a new single-stranded DNA virus infecting the cosmopolitan marine diatom Chaetoceros debilis. Aquat. Microb. Ecol. 2008, 50, 103–112. [Google Scholar] [CrossRef]
- Brussaard, C.P.D.; Noordeloos, A.A.; Sandaa, R.A.; Heldal, M.; Bratbak, G. Discovery of a dsRNA virus infecting the marine photosynthetic protist Micromonas pusilla. Virology 2004, 319, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, K.; Tomaru, Y.; Katanozaka, N.; Shirai, Y.; Nishida, K.; Itakura, S.; Yamaguchi, M. Isolation and Characterization of a Novel Single-Stranded RNA Virus Infecting the Bloom-Forming Diatom Rhizosolenia setigera. Appl. Environ. Microbiol. 2004, 70, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Tomaru, Y.; Katanozaka, N.; Nishida, K.; Shirai, Y.; Tarutani, K.; Yamaguchi, M.; Nagasaki, K. Isolation and characterization of two distinct types of HcRNAV, a single-stranded RNA virus infecting the bivalve-killing microalga Heterocapsa circularisquama. Aquat. Microb. Ecol. 2004, 34, 207–218. [Google Scholar] [CrossRef]
- Cottrell, M.T.; Suttle, C.A. Dynamics of lytic virus infecting the photosynthetic marine picoflagellate Micromonas pusilla. Limnol. Oceanogr. 1995, 40, 730–739. [Google Scholar] [CrossRef]
- Van Etten, J.; Agarkova, I.; Dunigan, D.; Tonetti, M.; De Castro, C.; Duncan, G. Chloroviruses Have a Sweet Tooth. Viruses 2017, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in Aquatic Ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, A.G.; Jackson, G.A. Viral dynamics: A model of the effects of size, shape, motion and abundance of single-celled planktonic organisms and other particles. Mar. Ecol. Prog. Ser. 1992, 89, 103–116. [Google Scholar] [CrossRef]
- Tarutani, K.; Nagasaki, K.; Yamaguchi, M. Virus adsorption process determines virus susceptibility in Heterosigma akashiwo (Raphidophyceae). Aquat. Microb. Ecol. 2006, 42, 209–213. [Google Scholar] [CrossRef]
- Nagasaki, K.; Yamaguchi, M. Intra-species host specificity of HaV (Heterosigma akashiwo virus) clones. Aquat. Microb. Ecol. 1998, 14, 109–112. [Google Scholar] [CrossRef]
- Tomaru, Y.; Takao, Y.; Suzuki, H.; Nagumo, T.; Koike, K.; Nagasaki, K. Isolation and Characterization of a Single-Stranded DNA Virus Infecting Chaetoceros lorenzianus Grunow. Appl. Environ. Microbiol. 2011, 77, 5285–5293. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.; Bratbak, G.; Heldal, M. Isolation and Characterization of a Virus Infecting Phaeocystis Pouchetii (prymnesiophyceae) 1. J. Phycol. 1996, 32, 923–927. [Google Scholar] [CrossRef]
- Müller, D.G.; Schmid, C.E. Intergeneric Infection and Persistence of Ectocarpus Virus DNA in Kuckuckia (Phaeophyceae, Ectocarpales). Bot. Mar. 1996, 39, 401–406. [Google Scholar] [CrossRef]
- Müller, D.G.; Parodi, E. Transfer of a marine DNA virus from Ectocarpus Feldmannia (Ectocarpales, Phaeophyceae): Aberrant symptoms and restitution of the host. Protoplasma 1993, 175, 121–125. [Google Scholar] [CrossRef]
- Müller, D.G.; Sengco, M.; Wolf, S.; Bräutigam, M.; Schmid, C.E.; Kapp, M.; Knippers, R. Comparison of two DNA Viruses Infecting the Marine Brown Algae Ectocarpus Siliculosus and E. Fasciculatus. J. Gen. Virol. 1996, 77, 2329–2333. [Google Scholar] [CrossRef] [PubMed]
- Maier, I.; Rometsch, E.; Wolf, S.; Kapp, M.; Müller, D.G.; Kawai, H. Passage of a Marine Brown Algal Dna Virus from Ectocarpus Fasciculatus (ectocarpales, Phaeophyceae) to Myriotrichia Clavaeformis (dictyosiphonales, Phaeophyceae): Infection Symptoms and Recovery 1. J. Phycol. 1997, 33, 838–844. [Google Scholar] [CrossRef]
- Corte, D.D.; Sintes, E.; Winter, C.; Yokokawa, T.; Reinthaler, T.; Herndl, G.J. Links between viral and prokaryotic communities throughout the water column in the (sub)tropical Atlantic Ocean. ISME J. 2010, 4, 1431–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angly, F.; Youle, M.; Nosrat, B.; Srinagesh, S.; Rodriguez-Brito, B.; McNairnie, P.; Deyanat-Yazdi, G.; Breitbart, M.; Rohwer, F. Genomic analysis of multiple Roseophage SIO1 strains. Environ. Microbiol. 2009, 11, 2863–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.H. Bacteriophages; Interscience Publishers, Inc.: New York, NY, USA, 1959. [Google Scholar]
- Thomas, R.; Grimsley, N.; Escande, M.; Subirana, L.; Derelle, E.; Moreau, H. Acquisition and maintenance of resistance to viruses in eukaryotic phytoplankton populations: Viral resistance in Mamiellales. Environ. Microbiol. 2011, 13, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.; Brockhurst, M.A. Ecological and Evolutionary Benefits of Temperate Phage: What Does or Doesn’t Kill You Makes You Stronger. BioEssays 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, D.C.; Oke, J.; Malin, G.; Wilson, W.H. Coccolithovirus (Phycodnaviridae): Characterisation of a new large dsDNA algal virus that infects Emiliana huxleyi. Arch. Virol. 2002, 147, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.G.; Kapp, M.; Knippers, R. Viruses in Marine Brown Algae. In Advances in Virus Research; Maramorosch, K., Murphy, F.A., Shatkin, A.J., Eds.; Academic Press: New York, NY, USA, 1998; Volume 50, pp. 49–67. [Google Scholar]
- Stevens, K.; Weynberg, K.; Bellas, C.; Brown, S.; Brownlee, C.; Brown, M.T.; Schroeder, D.C. A Novel Evolutionary Strategy Revealed in the Phaeoviruses. PLoS ONE 2014, 9, e86040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeown, D.; Schroeder, J.; Stevens, K.; Peters, A.; Sáez, C.; Park, J.; Rothman, M.; Bolton, J.; Brown, M.; Schroeder, D.; et al. Phaeoviral Infections Are Present in Macrocystis, Ecklonia and Undaria (Laminariales) and Are Influenced by Wave Exposure in Ectocarpales. Viruses 2018, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A.B. Ecological, Evolutionary, and Geochemical Consequences of Viral Infection in Cyanobacteria and Eukaryotic Algae. In Viral Ecology; Hurst, C.J., Ed.; Academic Press: New York, NY, USA, 2000; ISBN 978-0-12-362675-2. [Google Scholar]
- Weinbauer, M.G.; Höfle, M.G. Size-specific mortality of lake bacterioplankton by natural virus communities. Aquat. Microb. Ecol. 1998, 15, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Jacquet, S.; Grimsley, N.; Moreau, H. Strategies and mechanisms of resistance to viruses in photosynthetic aquatic microorganisms. Adv. Oceanogr. Limnol. 2012, 3, 1–15. [Google Scholar] [CrossRef]
- Huheihel, M.; Ishanu, V.; Tal, J.; Arad, S.M. Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods 2002, 50, 189–200. [Google Scholar] [CrossRef]
- Evans, C.; Malin, G.; Wilson, W.H.; Liss, P.S. Infectious titres of Emiliania huxleyi virus 86 are reduced by exposure to millimolar dimethyl sulfide and acrylic acid. Limnol. Oceanogr. 2006, 51, 2468–2471. [Google Scholar] [CrossRef]
- Jacobsen, A.; Larsen, A.; Martínez-Martínez, J.; Verity, P.G.; Frischer, M.E. Susceptibility of colonies and colonial cells of Phaeocystis pouchetii (Haptophyta) to viral infection. Aquat. Microb. Ecol. 2007, 48, 105–112. [Google Scholar] [CrossRef]
- Hamm, C.; Simson, D.; Merkel, R.; Smetacek, V. Colonies of Phaeocystis globosa are protected by a thin but tough skin. Mar. Ecol. Prog. Ser. 1999, 187, 101–111. [Google Scholar] [CrossRef]
- Ruardij, P.; Veldhuis, M.J.W.; Brussaard, C.P.D. Modeling the bloom dynamics of the polymorphic phytoplankter Phaeocystis globosa: Impact of grazers and viruses. Harmful Algae 2005, 4, 941–963. [Google Scholar] [CrossRef]
- Frickel, J.; Theodosiou, L.; Becks, L. Rapid evolution of hosts begets species diversity at the cost of intraspecific diversity. Proc. Natl. Acad. Sci. USA 2017, 114, 11193–11198. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Tomaru, Y. Coculture with marine bacteria confers resistance to complete viral lysis of diatom cultures. Aquat. Microb. Ecol. 2014, 73, 69–80. [Google Scholar] [CrossRef]
- Frada, M.; Probert, I.; Allen, M.J.; Wilson, W.H.; Vargas, C. The “Cheshire Cat” escape strategy of the coccolithophore Emiliania huxleyi in response to viral infection. Proc. Natl. Acad. Sci. USA 2008, 105, 15944–15949. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, K.; Tomaru, Y.; Tarutani, K.; Katanozaka, N.; Yamanaka, S.; Tanabe, H.; Yamaguchi, M. Growth Characteristics and Intraspecies Host Specificity of a Large Virus Infecting the Dinoflagellate Heterocapsa circularisquama. Appl. Environ. Microbiol. 2003, 69, 2580–2586. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, H.; Tomaru, Y.; Takao, Y.; Shirai, Y.; Nagasaki, K. Diverse responses of the bivalve-killing dinoflagellate Heterocapsa circularisquama to infection by a single-stranded RNA virus. Appl. Environ. Microbiol. 2008, 74, 3105–3111. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, H.; Tomaru, Y.; Takao, Y.; Shirai, Y.; Nagasaki, K. Intraspecies Host Specificity of a Single-Stranded RNA Virus Infecting a Marine Photosynthetic Protist Is Determined at the Early Steps of Infection. J. Virol. 2007, 81, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Tomaru, Y.; Mizumoto, H.; Nagasaki, K. Virus resistance in the toxic bloom-forming dinoflagellate Heterocapsa circularisquama to single-stranded RNA virus infection. Environ. Microbiol. 2009, 11, 2915–2923. [Google Scholar] [CrossRef] [PubMed]
- González, J.M.; Suttle, C.A. Grazing by marine nanoflagellates on viruses and virus-sized particles: Ingestion and digestion. Mar. Ecol. Prog. Ser. 1993, 94, 1–10. [Google Scholar] [CrossRef]
- Hadas, E.; Marie, D.; Shpigel, M.; Ilan, M. Virus predation by sponges is a new nutrient-flow pathway in coral reef food webs. Limnol. Oceanogr. 2006, 51, 1548–1550. [Google Scholar] [CrossRef] [Green Version]
- Mojica, K.D.A.; Brussaard, C.P.D. Factors affecting virus dynamics and microbial host-virus interactions in marine environments. FEMS Microbiol. Ecol. 2014, 89, 495–515. [Google Scholar] [CrossRef] [PubMed]
- Sheik, A.R.; Brussaard, C.P.D.; Lavik, G.; Lam, P.; Musat, N.; Krupke, A.; Littmann, S.; Strous, M.; Kuypers, M.M.M. Responses of the coastal bacterial community to viral infection of the algae Phaeocystis globosa. ISME J. 2014, 8, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Scola, B.L.; Desnues, C.; Pagnier, I.; Robert, C.; Barrassi, L.; Fournous, G.; Merchat, M.; Suzan-Monti, M.; Forterre, P.; Koonin, E.; et al. The virophage as a unique parasite of the giant mimivirus. Nature 2008, 455, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Wootton, J.T. The Nature and Consequences of Indirect Effects in Ecological Communities. Annu. Rev. Ecol. Syst. 1994, 25, 443–466. [Google Scholar] [CrossRef]
- Weinbauer, M.G.; Hornák, K.; Jezbera, J.; Nedoma, J.; Dolan, J.R.; Šimek, K. Synergistic and antagonistic effects of viral lysis and protistan grazing on bacterial biomass, production and diversity. Environ. Microbiol. 2007, 9, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Sandaa, R.A.; Pree, B.; Larsen, A.; Våge, S.; Töpper, B.; Töpper, J.; Thyrhaug, R.; Thingstad, T. The Response of Heterotrophic Prokaryote and Viral Communities to Labile Organic Carbon Inputs Is Controlled by the Predator Food Chain Structure. Viruses 2017, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- DeLong, J.P.; Al-Ameeli, Z.; Lyon, S.; Etten, J.L.V.; Dunigan, D.D. Size-dependent Catalysis of Chlorovirus Population Growth by A Messy Feeding Predator. Microb. Ecol. 2018, 75, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Zwirglmaier, K.; Spence, E.; Zubkov, M.V.; Scanlan, D.J.; Mann, N.H. Differential grazing of two heterotrophic nanoflagellates on marine Synechococcus strains. Environ. Microbiol. 2002, 11, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Corinaldesi, C.; Dell’Anno, A.; Fuhrman, J.A.; Middelburg, J.J.; Noble, R.T.; Suttle, C.A. Marine viruses and global climate change. FEMS Microbiol. Rev. 2011, 35, 993–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brussaard, C.P.D. Viral Control of Phytoplankton Populations—A Review. J. Eukaryot. Microbiol. 2004, 51, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Tomaru, Y. Effects of temperature and salinity on diatom cell lysis by DNA and RNA viruses. Aquat. Microb. Ecol. 2017, 79, 79–83. [Google Scholar] [CrossRef]
- Baudoux, A.C.; Brussaard, C.P.D. Characterization of different viruses infecting the marine harmful algal bloom species Phaeocystis globosa. Virology 2005, 341, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsen, A. Populationsdynamik Zwischen der Grünalge Chlorella variabilis und dem Chlorovirus Pbcv-1 in Abhängigkeit Verschiedener Temperaturen. Bachelor’s Thesis, Kiel University, Kiel, Germany, 2016. [Google Scholar]
- Frickel, J.; Sieber, M.; Becks, L. Eco-evolutionary dynamics in a coevolving host-virus system. Ecol. Lett. 2016, 19, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A.; Chen, F. Mechanisms and Rates of Decay of Marine Viruses in Seawater. Appl. Environ. Microbiol. 1992, 58, 3721–3729. [Google Scholar] [PubMed]
- Bratbak, G.; Jacobsen, A.; Heldal, M. Viral lysis of Phaeocystis pouchetii and bacterial secondary production. Aquat. Microb. Ecol. 1998, 16, 11–16. [Google Scholar] [CrossRef]
- Thyrhaug, R.; Larsen, A.; Brussaard, C.P.D.; Bratbak, G. Cell Cycle Dependent Virus Production in Marine Phytoplankton 1. J. Phycol. 2002, 38, 338–343. [Google Scholar] [CrossRef]
- Juneau, P.; Lawrence, J.E.; Suttle, C.A.; Harrison, P.J. Effects of viral infection on photosynthetic processes in the bloom-forming alga Heterosigma akashiwo. Aquat. Microb. Ecol. 2003, 31, 9–17. [Google Scholar] [CrossRef]
- Lawrence, J.E.; Suttle, C.A. Effect of viral infection on sinking rates of Heterosigma akashiwo and its implications for bloom termination. Aquat. Microb. Ecol. 2004, 37, 1–7. [Google Scholar] [CrossRef]
- Jacquet, S.; Bratbak, G. Effects of ultraviolet radiation on marine virus-phytoplankton interactions. FEMS Microbiol. Ecol. 2003, 44, 279–289. [Google Scholar] [CrossRef]
- Tomaru, Y.; Tanabe, H.; Yamanaka, S.; Nagasaki, K. Effects of temperature and light on stability of microalgal viruses, HaV, HcV and HcRNAV. Plankton Biol. Ecol. 2005, 52, 1–6. [Google Scholar] [CrossRef]
- Long, A.M.; Short, S.M. Seasonal determinations of algal virus decay rates reveal overwintering in a temperate freshwater pond. ISME J. 2016, 10, 1602–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jover, L.F.; Effler, T.C.; Buchan, A.; Wilhelm, S.W.; Weitz, J.S. The elemental composition of virus particles: Implications for marine biogeochemical cycles. Nat. Rev. Microbiol. 2014, 12, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Clerissi, C.; Grimsley, N.; Subirana, L.; Maria, E.; Oriol, L.; Ogata, H.; Moreau, H.; Desdevises, Y. Prasinovirus distribution in the Northwest Mediterranean Sea is affected by the environment and particularly by phosphate availability. Virology 2014, 466–467, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Clasen, J.L.; Elser, J.J. The effect of host Chlorella NC64A carbon: Phosphorus ratio on the production of Paramecium bursaria Chlorella Virus-1. Freshw. Biol. 1995, 52, 112–122. [Google Scholar] [CrossRef]
- Bratbak, G.; Egge, J.K.; Heldal, M. Viral mortality of the marine alga Emiliania huxleyi (Haptophyceae) and termination of algal blooms. Mar. Ecol. Prog. Ser. 1993, 93, 39–48. [Google Scholar] [CrossRef]
- Maat, D.S.; Crawfurd, K.J.; Timmermans, K.R.; Brussaard, C.P.D. Elevated CO2 and Phosphate Limitation Favor Micromonas pusilla through Stimulated Growth and Reduced Viral Impact. Appl. Environ. Microbiol. 2014, 80, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Bachy, C.; Charlesworth, C.J.; Chan, A.M.; Finke, J.F.; Wong, C.-H.; Wei, C.-L.; Sudek, S.; Coleman, M.L.; Suttle, C.A.; Worden, A.Z. Transcriptional responses of the marine green alga Micromonas pusilla and an infecting prasinovirus under different phosphate conditions. Environ. Microbiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bratbak, G.; Jacobsen, A.; Heldal, M.; Nagasaki, K.; Thingstad, F. Virus production in Phaeocystis pouchetii and its relation to host cell growth and nutrition. Aquat. Microb. Ecol. 1998, 16, 1–9. [Google Scholar] [CrossRef]
- Maat, D.S.; Brussaard, C.P.D. Both phosphorus-and nitrogen limitation constrain viral proliferation in marine phytoplankton. Aquat. Microb. Ecol. 2016, 77, 87–97. [Google Scholar] [CrossRef]
- Finke, J.F.; Hunt, B.P.V.; Winter, C.; Carmack, E.C.; Suttle, C.A. Nutrients and Other Environmental Factors Influence Virus Abundances across Oxic and Hypoxic Marine Environments. Viruses 2017, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Weinbauer, M.; Bettarel, Y.; Cattaneo, R.; Luef, B.; Maier, C.; Motegi, C.; Peduzzi, P.; Mari, X. Viral ecology of organic and inorganic particles in aquatic systems: Avenues for further research. Aquat. Microb. Ecol. 2009, 57, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Baudoux, A.-C.; Noordeloos, A.A.M.; Veldhuis, M.J.W.; Brussaard, C.P.D. Virally induced mortality of Phaeocystis globosa during two spring blooms in temperate coastal waters. Aquat. Microb. Ecol. 2006, 44, 207–217. [Google Scholar] [CrossRef]
- Brussaard, C.P.D.; Mari, X.; Van Bleijswijk, J.D.L.; Veldhuis, M.J.W. A mesocosm study of Phaeocystis globosa (Prymnesiophyceae) population dynamics: II. Significance for the microbial community. Harmful Algae 2005, 4, 875–893. [Google Scholar] [CrossRef]
- Mari, X.; Kerros, M.-E.; Weinbauer, M.G. Virus Attachment to Transparent Exopolymeric Particles along Trophic Gradients in the Southwestern Lagoon of New Caledonia. Appl. Environ. Microbiol. 2007, 73, 5245–5252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, J.B.; Larsen, A.; Thyrhaug, R.; Bratbak, G.; Sandaa, R.A. Response of marine viral populations to a nutrient induced phytoplankton bloom at different pCO2 levels. Biogeosciences 2008, 5, 523–533. [Google Scholar] [CrossRef]
- Highfield, A.; Joint, I.; Gilbert, J.A.; Crawfurd, K.J.; Schroeder, D.C. Change in Emiliania huxleyi Virus Assemblage Diversity but Not in Host Genetic Composition during an Ocean Acidification Mesocosm Experiment. Viruses 2017, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gao, K.; Beardall, J. Viral attack exacerbates the susceptibility of a bloom-forming alga to ocean acidification. Glob. Chang. Biol. 2015, 21, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Guixa-Boixareu, N.; Calderón-Paz, J.; Heldal, M.; Bratbak, G.; Pedrós-Alió, C. Viral lysis and bacterivory as prokaryotic loss factors along a salinity gradient. Aquat. Microb. Ecol. 1996, 11, 215–227. [Google Scholar] [CrossRef] [Green Version]
- Edwards, K.F.; Steward, G.F. Data from: Host traits drive viral life histories across phytoplankton viruses. Am. Nat. 2018, 191, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Martiny, J.B.H.; Riemann, L.; Marston, M.F.; Middelboe, M. Antagonistic Coevolution of Marine Planktonic Viruses and Their Hosts. Annu. Rev. Mar. Sci. 2014, 6, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Monier, A.; Pagarete, A.; de Vargas, C.; Allen, M.J.; Read, B.; Claverie, J.M.; Ogata, H. Horizontal gene transfer of an entire metabolic pathway between a eukaryotic alga and its DNA virus. Genome Res. 2009, 19, 1441–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacquet, S.; Heldal, M.; Iglesias-Rodriguez, D.; Larsen, A.; Wilson, W.; Bratbak, G. Flow cytometric analysis of an Emiliana huxleyi bloom terminated by viral infection. Aquat. Microb. Ecol. 2002, 27, 111–124. [Google Scholar] [CrossRef]
- Derelle, E.; Yau, S.; Moreau, H.; Grimsley, N.H. Prasinovirus Attack of Ostreococcus Is Furtive by Day but Savage by Night. J. Virol. 2017, 92. [Google Scholar] [CrossRef]
- Furuta, M.; Schrader, J.O.; Schrader, H.S.; Kokjohn, T.A.; Nyaga, S.; McCullough, A.K.; Lloyd, R.S.; Burbank, D.E.; Landstein, D.; Lane, L.; et al. Chlorella virus PBCV-1 encodes a homolog of the bacteriophage T4 UV damage repair gene denV. Appl. Environ. Microbiol. 1997, 63, 1551–1556. [Google Scholar] [PubMed]
- Lawrence, J.E.; Chan, A.M.; Suttle, C.A. Viruses causing lysis of the toxic bloom-forming alga Heterosigma akashiwo (Raphidophyceae) are widespread in coastal sediments of British Columbia, Canada. Limnol. Oceanogr. 2002, 47, 545–550. [Google Scholar] [CrossRef]
- Thyrhaug, R.; Larsen, A.; Thingstad, T.F.; Bratbak, G. Stable coexistence in marine algal host-virus systems. Mar. Ecol. Prog. Ser. 2003, 254, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Monier, A.; Larsen, J.B.; Sandaa, R.A.; Bratbak, G.; Claverie, J.M.; Ogata, H. Marine mimivirus relatives are probably large algal viruses. Virol. J. 2008, 5, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Suttle, C.A. Evolutionary Relationships among Large Double-Stranded DNA Viruses That Infect Microalgae and Other Organisms as Inferred from DNA Polymerase Genes. Virology 1996, 219, 170–178. [Google Scholar] [CrossRef] [PubMed]


| Approach | Method | Examples * |
|---|---|---|
| Comparative studies | ||
| Sampling of communities over time and correlation with changes in extrinsic factors | [95] | |
| Resurrection ecology, correlation of abundances with changes in the environment | [119] | |
| Experimental studies | ||
| Measurement of virus life cycle traits under different conditions | [83,85,100,101] | |
| Resurrection ecology, isolation of living viruses and measurement of life cycle traits under different conditions | ||
| Experimental evolution studies | ||
| Virus evolution to different conditions (requires constant host) | ||
| Host-virus coevolution under different conditions | [87,120] | |
| Virus (co)evolution in communities under different conditions | [65] | |
| Modeling | ||
| Virus population events across different conditions | [87] | |
| Genomics | ||
| Viromics to check for absence/presence of viruses across different conditions | [18] | |
| Genomics and phylogenetic trees to decipher evolution and past population events (bottlenecks, extinctions, migrations, etc.) | [115,121,122] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horas, E.L.; Theodosiou, L.; Becks, L. Why Are Algal Viruses Not Always Successful? Viruses 2018, 10, 474. https://doi.org/10.3390/v10090474
Horas EL, Theodosiou L, Becks L. Why Are Algal Viruses Not Always Successful? Viruses. 2018; 10(9):474. https://doi.org/10.3390/v10090474
Chicago/Turabian StyleHoras, Elena L., Loukas Theodosiou, and Lutz Becks. 2018. "Why Are Algal Viruses Not Always Successful?" Viruses 10, no. 9: 474. https://doi.org/10.3390/v10090474
APA StyleHoras, E. L., Theodosiou, L., & Becks, L. (2018). Why Are Algal Viruses Not Always Successful? Viruses, 10(9), 474. https://doi.org/10.3390/v10090474