Characterization of a Botybirnavirus Conferring Hypovirulence in the Phytopathogenic Fungus Botryosphaeria dothidea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates and Culture Conditions
2.2. Extraction and Purification of dsRNA
2.3. cDNA Synthesis and Molecular Cloning
2.4. Sequence Analysis, Alignment, and Phylogenetic Analysis
2.5. Virion Purification
2.6. Vertical and Horizontal Transmission
2.7. Biological Testing
3. Results
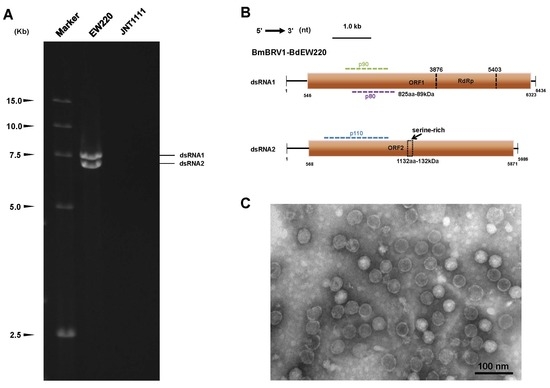
3.1. Biological Characteristics of Strain EW220 and the Detection of dsRNA
3.2. Genetic Analysis of dsRNAs
3.3. Phylogenetic Analysis of the dsRNA Virus
3.4. Virus Particles
3.5. Structural Proteins of the Virus
3.6. Vertical and Horizontal Transmission of BmBRV1-BdEW220
3.7. Influence of BdBRV1 on the Biological Properties of B. dothidea
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghabrial, S.A.; Caston, J.R.; Jiang, D.; Nibert, M.L.; Suzuki, N. 50-plus years of fungal viruses. Virology 2015, 479, 356–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghabrial, S.A.; Suzuki, N. Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 2009, 47, 353–384. [Google Scholar] [CrossRef]
- Pearson, M.N.; Beever, R.E.; Boine, B.; Arthur, K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 2009, 10, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.T.; Jiang, D.H. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 2014, 52, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Donaire, L.; Pagán, I.; Ayllón, M.A. Characterization of Botrytis cinerea negative-stranded RNA virus 1, a new mycovirus related to plant viruses, and a reconstruction of host pattern evolution in negative-sense ssRNA viruses. Virology 2016, 499, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Xie, J.T.; Cheng, J.S.; Fu, Y.P.; Li, G.Q.; Yi, X.H.; Jiang, D.H. Fungal negative-stranded RNA virus that is related to bornaviruses and nyaviruses. Proc. Natl. Acad. Sci. USA 2014, 111, 12205–12210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzano, S.Y.L.; Nelson, B.D.; Ajayi-Oyetunde, O.; Bradley, C.A.; Hughes, T.J.; Hartman, G.L.; Eastburn, D.M.; Domier, L.L. Identification of diverse mycoviruses through metatranscriptomics characterization of the viromes of five major fungal plant pathogens. J. Virol. 2016, 90, 6846–6863. [Google Scholar] [CrossRef]
- Mu, F.; Xie, J.T.; Cheng, S.F.; You, M.P.; Barbetti, M.J.; Jia, J.C.; Wang, Q.Q.; Cheng, J.S.; Fu, Y.P.; Chen, T.; et al. Virome characterization of a collection of Sclerotinia sclerotiorum from Australia. Front. Microbiol. 2018, 8, 2540. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, H.; Wang, S.C.; Chen, X.G.; Qiu, D.W.; Kondo, H.; Guo, L.H. Evidence for a novel negative-stranded RNA mycovirus isolated from the plant pathogenic fungus Fusarium graminearum. Virology 2018, 518, 232. [Google Scholar] [CrossRef]
- Yu, X.; Li, B.; Fu, Y.P.; Jiang, D.H.; Ghabrial, S.A.; Li, G.Q.; Peng, Y.L.; Xie, J.T.; Cheng, J.S.; Huang, J.B.; et al. A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc. Natl. Acad. Sci. USA 2010, 107, 8387–8392. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.D.; Jin, F.Y.; Zhang, J.; Yang, L.; Jiang, D.H.; Li, G.Q. Characterization of a novel bipartite double-stranded RNA mycovirus conferring hypovirulence in the phytopathogenic fungus Botrytis porri. J. Virol. 2012, 86, 6605–6619. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Wang, Q.Q.; Cheng, J.S.; Fu, Y.P.; Jiang, D.H.; Xie, J.T. Molecular characterization of a bipartite double-stranded RNA virus and its satellite-like RNA co-infecting the phytopathogenic fungus Sclerotinia sclerotiorum. Front. Microbiol. 2015, 6, 406. [Google Scholar] [CrossRef] [PubMed]
- Ran, H.C.; Liu, L.J.; Li, B.; Cheng, J.S.; Fu, Y.P.; Jiang, D.H.; Xie, J.T. Co-infection of a hypovirulent isolate of Sclerotinia sclerotiorum with a new botybirnavirus and a strain of a mitovirus. Virol. J. 2016, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Fu, M.; Hong, N.; Zhai, L.F.; Xiao, F.; Wang, G.P. Characterization of a novel botybirnavirus isolated from a phytopathogenic Alternaria fungus. Arch. Virol. 2017, 162, 3907. [Google Scholar] [CrossRef] [PubMed]
- Marzano, S.Y.L.; Domier, L.L. Novel mycoviruses discovered from metatranscriptomics survey of soybean phyllosphere phytobiomes. Virus Res. 2016, 213, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.R.; Li, C.; Cai, L.; Fang, S.G.; Zheng, L.M.; Yan, F.; Zhang, S.B.; Liu, Y. The complete genomic sequence of a novel botybirnavirus isolated from a phytopathogenic Bipolaris maydis. Virus Genes 2018, 54, 733–736. [Google Scholar] [CrossRef]
- Tran, T.T.; Li, H.; Nguyen, D.Q.; Jones, M.G.K.; Wylie, S.J. Co-infection with three mycoviruses stimulates growth of a Monilinia fructicola isolate on nutrient medium, but does not induce hypervirulence in a natural host. Viruses 2019, 11, 89. [Google Scholar] [CrossRef]
- Anagnostakis, S.L. Biological control of chestnut blight. Science 1982, 215, 466–471. [Google Scholar] [CrossRef]
- MacDonald, W.L.; Fulbdght, D.W. Biological control of chestnut blight: Use and limitations of transmissible hypovirulence. Plant Dis. 1991, 75, 656–661. [Google Scholar] [CrossRef]
- Kamaruzzaman, M.; He, G.Y.; Wu, M.D.; Zhang, J.; Yang, L.; Chen, W.D.; Li, G.Q. A novel partitivirus in the hypovirulent isolate QT5-19 of the plant pathogenic fungus Botrytis cinerea. Viruses 2019, 11, 24. [Google Scholar] [CrossRef]
- Wang, L.P.; Jiang, J.J.; Wang, Y.F.; Hong, N.; Zhang, F.P.; Xu, W.X.; Wang, G.P. Hypovirulence of the phytopathogenic fungus Botryosphaeria dothidea: Association with a coinfecting chrysovirus and a partitivirus. J. Virol. 2014, 88, 7517–7527. [Google Scholar] [CrossRef]
- Xiao, X.Q.; Cheng, J.S.; Tang, J.H.; Fu, Y.P.; Jiang, D.H.; Baker, T.S.; Ghabrial, S.A.; Xie, J.T. A novel partitivirus that confers hypovirulence on plant pathogenic fungi. J. Virol. 2014, 88, 10120–10133. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, B.; Fu, Y.P.; Xie, J.T.; Cheng, J.S.; Ghabrial, S.A.; Li, G.Q.; Yi, X.H.; Jiang, D.H. Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc. Natl. Acad. Sci. USA 2013, 110, 1452–1457. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.F.; Xiang, J.; Zhang, M.X.; Fu, M.; Yang, Z.K.; Hong, N.; Wang, G.P. Characterization of a novel double-stranded RNA mycovirus conferring hypovirulence from the phytopathogenic fungus Botryosphaeria dothidea. Virology 2016, 493, 75–85. [Google Scholar] [CrossRef]
- Zhai, L.F.; Zhang, M.X.; Hong, N.; Xiao, F.; Fu, M.; Xiang, J.; Wang, G.P. Identification and characterization of a novel hepta-segmented dsRNA virus from the phytopathogenic fungus Colletotrichum fructicola. Front. Microbiol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Phillips, A.J.L.; Li, X.; Hyde, K.D. Botryosphaeriaceae: Current status of genera and species. Mycosphere 2016, 7, 1001–1073. [Google Scholar] [CrossRef]
- Marsberg, A.; Kemler, M.; Jami, F.; Nagel, J.H.; Postma-Smidt, A.; Naidoo, S.; Wingfield, M.J.; Crous, P.W.; Spatafora, J.W.; Hesse, C.N.; et al. Botryosphaeria dothidea: A latent pathogen of global importance to woody plant health. Mol. Plant Pathol. 2017, 18, 477–488. [Google Scholar] [CrossRef]
- Tang, W.; Ding, Z.; Zhou, Z.Q.; Wang, Y.Z.; Guo, L.Y. Phylogenetic and pathogenic analyses show that the causal agent of apple ring rot in China is Botryosphaeria dothidea. Plant Dis. 2012, 96, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, C.S.; Ju, L.L.; Zhang, R.; Biggs, A.R.; Tanaka, E.; Li, B.Z.; Sun, G.Y. Multiple locus genealogies and phenotypic characters reappraise the causal agents of apple ring rot in China. Fungal Divers. 2015, 71, 215–231. [Google Scholar] [CrossRef]
- Zhai, L.F.; Zhang, M.X.; Lv, G.; Chen, X.R.; Jia, N.N.; Hong, N.; Wang, G.P. Biological and molecular characterization of four Botryosphaeria species isolated from pear plants showing stem wart and stem canker in China. Plant Dis. 2014, 98, 716–726. [Google Scholar] [CrossRef]
- Yan, J.Y.; Xie, Y.; Zhang, W.; Wang, Y.; Liu, J.K.; Hyde, K.D.; Seem, R.C.; Zhang, G.Z.; Wang, Z.Y.; Yao, S.W.; et al. Species of Botryosphaeriaceae involved in grapevine dieback in China. Fungal Divers. 2013, 61, 221–236. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, T.; Guo, L.Y. Characterization of a novel strain of Botryosphaeria dothidea chrysovirus 1 from the apple white rot pathogen Botryosphaeria dothidea. Arch. Virol. 2017, 162, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.F.; Hong, N.; Zhang, M.X.; Wang, G.P. Complete dsRNA sequence of a novel victorivirus isolated from the pear stem wart fungus Botryosphaeria dothidea. Arch. Virol. 2015, 160, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Frisch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Lambden, P.R.; Cooke, S.J.; Caul, E.O.; Clarke, I.N. Cloning of noncultivatable human rotavirus by single primer amplification. J. Virol. 1992, 66, 1817–1822. [Google Scholar] [PubMed]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Zhang, X.; Nuss, D.L. A host dicer is required for defective viral RNA production and recombinant virus vector RNA instability for a positive sense RNA virus. Proc. Natl. Acad. Sci. USA 2008, 105, 16749–16754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buck, K.W.; Esteban, R.; Hillman, B.I. Narnaviridae. In Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses, 1st ed.; Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U., Ball, L.A., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2005; pp. 751–756. [Google Scholar]
- Bruns, H.A. Southern corn leaf blight: A story worth retelling. Agron. J. 2017, 109, 1218–1224. [Google Scholar] [CrossRef]
- Deng, F.; Xu, R.; Boland, G. Hypovirulence-associated double-stranded RNA from Sclerotinia homoeocarpa is conspecific with Ophiostoma novo-ulmi mitovirus 3a-Ld. Phytopathology 2003, 93, 1407–1414. [Google Scholar] [CrossRef]
- Liu, S.; Xie, J.T.; Cheng, J.S.; Li, B.; Chen, T.; Fu, Y.P.; Li, G.Q.; Wang, M.Q.; Jin, H.N.; Hu, W.H.; et al. A Fungal DNA virus infects a mycophagous insect and utilizes it as a transmission vector. Proc. Natl. Acad. Sci. USA 2016, 113, 12803–12808. [Google Scholar] [CrossRef] [PubMed]
- Buck, K.W. Molecular variability of viruses of fungi. In Molecular Variability of Fungal Pathogens; Bridge, P., Couteaudier, Y., Clarkson, J., Eds.; CAB Intl: Wallingford, Oxfordshire, UK, 1998; pp. 53–72. [Google Scholar]
- Ejmal, M.A.; Holland, D.J.; MacDiarmid, R.M.; Pearson, M.N. The effect of Aspergillus thermomutatus Chrysovirus 1 on the biology of three Aspergillus species. Viruses 2018, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Melzer, M.; Ikeda, S.S.; Boland, G.J. Interspecific transmission of double-stranded RNA and hypovirulence from Sclerotinia sclerotiorum to S. minor. Phytopathology 2002, 92, 780–784. [Google Scholar] [CrossRef]
- Liu, Y.C.; Linder-Basso, D.; Hillman, B.I.; Kaneko, S.; Milgroom, M.G. Evidence for interspecies transmission of viruses in natural populations of filamentous fungi in the genus Cryphonectria. Mol. Ecol. 2003, 12, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Vainio, E.J.; Hakanpää, J.; Dai, Y.C.; Hansen, E.; Korhonen, K.; Hantula, J. Species of Heterobasidion host a diverse pool of partitiviruses with global distribution and interspecies transmission. Fungal Biol. 2011, 115, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Anzola, J.V.; Xu, Z.K.; Asamizu, T.; Nuss, D.L. Segment-specific inverted repeats found adjacent to conserved terminal sequences in wound tumor virus genome and defective interfering RNAs. Proc. Natl. Acad. Sci. USA 1987, 84, 8301–8305. [Google Scholar] [CrossRef]
- Wei, C.Z.; Saki, H.; Iwanami, T.; Matsumoto, N.; Ohtsu, Y. Molecular characterization of dsRNA segments 2 and 5 and electron microscopy of a novel reovirus from a hypovirulent isolate, W370, of the plant pathogen Rosellinia necatrix. J. Gen. Virol. 2003, 84, 2431–2437. [Google Scholar] [CrossRef]







| Strain | Origin | BmBRV1-BdEW220 |
|---|---|---|
| EW220 | Pyrus pyrifolia, Wuhan, China | positive |
| JNT1111 | P. bretschneideri, Shanxi, China | negative |
| EW220-64 | A single-conidium isolate of EW220 | negative |
| EW220-64-T1 | EW220-64 in a pairing culture of EW220-64 and EW220 | positive |
| EW220-64-T2 | EW220-64 in a pairing culture of EW220-64 and EW220 | positive |
| EW220-64-T3 | EW220-64 in a pairing culture of EW220-64 and EW220 | positive |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, L.; Yang, M.; Zhang, M.; Hong, N.; Wang, G. Characterization of a Botybirnavirus Conferring Hypovirulence in the Phytopathogenic Fungus Botryosphaeria dothidea. Viruses 2019, 11, 266. https://doi.org/10.3390/v11030266
Zhai L, Yang M, Zhang M, Hong N, Wang G. Characterization of a Botybirnavirus Conferring Hypovirulence in the Phytopathogenic Fungus Botryosphaeria dothidea. Viruses. 2019; 11(3):266. https://doi.org/10.3390/v11030266
Chicago/Turabian StyleZhai, Lifeng, Mengmeng Yang, Meixin Zhang, Ni Hong, and Guoping Wang. 2019. "Characterization of a Botybirnavirus Conferring Hypovirulence in the Phytopathogenic Fungus Botryosphaeria dothidea" Viruses 11, no. 3: 266. https://doi.org/10.3390/v11030266
APA StyleZhai, L., Yang, M., Zhang, M., Hong, N., & Wang, G. (2019). Characterization of a Botybirnavirus Conferring Hypovirulence in the Phytopathogenic Fungus Botryosphaeria dothidea. Viruses, 11(3), 266. https://doi.org/10.3390/v11030266