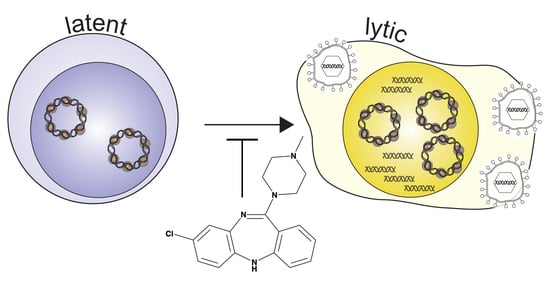
Inhibition of Epstein-Barr Virus Lytic Reactivation by the Atypical Antipsychotic Drug Clozapine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Chemical Treatments
2.3. Lytic Reactivation by RT-qPCR
2.4. Statistical Analysis
3. Results
3.1. Clozapine Blocked the Induction of EBV Lytic Genes
3.2. Dose-Dependent Inhibition by Clozapine
3.3. Clozapine Decreased EBV Lytic Induction by dAzaC and TPA
3.4. Metabolites of Clozapine
4. Discussion
4.1. Concentrations of Clozapine in Therapeutic Use
4.2. Metabolites of Clozapine
4.3. The Effects of Clozapine on Immune Cells
4.4. Mechanism of Action
4.5. Effect of Clozapine and Its Metabolites on Other Viruses
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Miller, G.; El-Guindy, A.; Countryman, J.; Ye, J.; Gradoville, L. Lytic Cycle Switches of Oncogenic Human Gammaherpesviruses1. Adv. Cancer Res. 2007, 97, 81–109. [Google Scholar] [PubMed]
- Luka, J.; Kallin, B.; Klein, G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology 1979, 94, 228–231. [Google Scholar] [CrossRef]
- Ben-Ssson, S.A.; Klein, G. Activation of the epstein-barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int. J. Cancer 1981, 28, 131–135. [Google Scholar] [CrossRef]
- Tuck, D.; Gradoville, L.; Daigle, D.; Gorres, K.; Wang’ondu, R.; Miller, G.; Schulz, V.; Ye, J. Valproic Acid Antagonizes the Capacity of Other Histone Deacetylase Inhibitors To Activate the Epstein-Barr Virus Lytic Cycle. J. Virol. 2011, 85, 5628–5643. [Google Scholar]
- Gorres, K.L.; Daigle, D.; Mohanram, S.; McInerney, G.E.; Lyons, D.E.; Miller, G. Valpromide Inhibits Lytic Cycle Reactivation of Epstein-Barr Virus. MBio 2016, 7, e00113. [Google Scholar] [CrossRef]
- Wenthur, C.J.; Lindsley, C.W. Classics in chemical neuroscience: Clozapine. ACS Chem. Neurosci. 2013, 4, 1018–1025. [Google Scholar] [CrossRef]
- Nucifora, F.C.; Mihaljevic, M.; Lee, B.J.; Sawa, A. Clozapine as a Model for Antipsychotic Development. Neurotherapeutics 2017, 14, 750–761. [Google Scholar] [CrossRef]
- Heston, L.; Rabson, M.; Brown, N.; Miller, G. New Epstein–Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature 1982, 295, 160–163. [Google Scholar] [CrossRef]
- Countryman, J.; Jenson, H.; Seibl, R.; Wolf, H.; Miller, G. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J. Virol. 1987, 61, 3672–3679. [Google Scholar] [PubMed]
- Kenney, S.; Holley-Guthrie, E.; Mar, E.C.; Smith, M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J. Virol. 1989, 63, 3878–3883. [Google Scholar]
- Buisson, M.; Manet, E.; Trescol-Biemont, M.C.; Gruffat, H.; Durand, B.; Sergeant, A. The Epstein-Barr virus (EBV) early protein EB2 is a posttranscriptional activator expressed under the control of EBV transcription factors EB1 and R. J. Virol. 1989, 63, 5276–5284. [Google Scholar] [PubMed]
- Himmelfarb, S.; Bhaduri-McIntosh, S.; Gradoville, L.; Heston, L.; Miller, G.; Ye, J.; Shedd, D.; Countryman, J. Stimulus Duration and Response Time Independently Influence the Kinetics of Lytic Cycle Reactivation of Epstein-Barr Virus. J. Virol. 2009, 83, 10694–10709. [Google Scholar]
- Centorrino, F.; Baldessarini, R.J.; Kando, J.C.; Frankenburg, F.R.; Volpicelli, S.A.; Flood, J.G. Clozapine and metabolites: concentrations in serum and clinical findings during treatment of chronically psychotic patients. J. Clin. Psychopharmacol. 1994, 14, 119–125. [Google Scholar] [CrossRef]
- Subramanian, S.; Völlm, B.A.; Huband, N. Clozapine dose for schizophrenia. Cochrane Database Syst. Rev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauri, M.C.; Volonteri, L.S.; Colasanti, A.; Fiorentini, A.; De Gaspari, I.F.; Bareggi, S.R. Clinical Pharmacokinetics of Atypical Antipsychotics A Critical Review of the Relationship Between Plasma Concentrations and Clinical Response. Clin. Pharmacokinet. 2007, 46, 359–388. [Google Scholar] [CrossRef]
- Stark, A.; Scott, J. A review of the use of clozapine levels to guide treatment and determine cause of death. Aust. New Zeal. J. Psychiatry 2012, 46, 816–825. [Google Scholar] [CrossRef]
- Heiser, P.; Enning, F.; Krieg, J.-C.; Vedder, H. Effects of haloperidol, clozapine and olanzapine on the survival of human neuronal and immune cells. J. Psychopharmacol. 2007, 21, 851–856. [Google Scholar] [CrossRef]
- Gardner, I.; Leeder, J.S.; Chin, T.; Zahid, N.; Uetrecht, J.P. A comparison of the covalent binding of clozapine and olanzapine to human neutrophils in vitro and in vivo. Mol. Pharmacol. 1998, 53, 999–1008. [Google Scholar]
- Chang, W.-H.; Lin, S.-K.; Lane, H.-Y.; Wei, F.-C.; Hu, W.-H.; Lam, Y.F.; Jann, M.W. Reversible metabolism of clozapine and clozapine N-oxide in schizophrenic patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1998, 22, 723–739. [Google Scholar] [CrossRef]
- Ghosh, A.; Chakraborty, K.; Mattoo, S.K. Newer molecules in the treatment of schizophrenia: A clinical update. Indian J. Pharmacol. 2011, 43, 105–112. [Google Scholar]
- Heusler, P.; Bruins Slot, L.; Tourette, A.; Tardif, S.; Cussac, D. The clozapine metabolite N-desmethylclozapine displays variable activity in diverse functional assays at human dopamine D 2 and serotonin 5-HT 1A receptors. Eur. J. Pharmacol. 2011, 669, 51–58. [Google Scholar] [CrossRef]
- Sur, C.; Mallorga, P.J.; Wittmann, M.; Jacobson, M.A.; Pascarella, D.; Williams, J.B.; Brandish, P.E.; Pettibone, D.J.; Scolnick, E.M.; Conn, P.J. N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl- D-aspartate receptor activity. Proc. Natl. Acad. Sci. USA 2003, 100, 13674–13679. [Google Scholar] [CrossRef]
- Kuoppamäki, M.; Syvälahti, E.; Hietala, J. Clozapine and N-desmethylclozapine are potent 5-HT1C receptor antagonists. Eur. J. Pharmacol. 1993, 245, 179–182. [Google Scholar] [CrossRef]
- Kuoppamiiki, M.; Liiddens, H.; Syviilahti, E. Effects of clozapine metabolites and chronic clozapine treatment on rat brain. Eur. J. Pharmacol. 1996, 314, 319–323. [Google Scholar]
- Numata, S.; Umehara, H.; Ohmori, T.; Hashimoto, R. Clozapine Pharmacogenetic Studies in Schizophrenia: Efficacy and Agranulocytosis. Front. Pharmacol. 2018, 9, 1049. [Google Scholar] [CrossRef]
- Wiciński, M.; Węclewicz, M.M. Clozapine-induced agranulocytosis/granulocytopenia. Curr. Opin. Hematol. 2018, 25, 22–28. [Google Scholar] [CrossRef]
- Chen, M.-L.; Wu, S.; Tsai, T.-C.; Wang, L.-K.; Tsai, F.-M. Regulation of macrophage immune responses by antipsychotic drugs. Immunopharmacol. Immunotoxicol. 2013, 35, 573–580. [Google Scholar] [CrossRef]
- Røge, R.; Møller, B.K.; Andersen, C.R.; Correll, C.U.; Nielsen, J. Immunomodulatory effects of clozapine and their clinical implications: What have we learned so far? Schizophr. Res. 2012, 140, 204–213. [Google Scholar] [CrossRef]
- Chen, M.-L.; Tsai, T.-C.; Wang, L.-K.; Lin, Y.-Y.; Tsai, Y.-M.; Lee, M.-C.; Tsai, F.-M. Clozapine inhibits Th1 cell differentiation and causes the suppression of IFN-γ production in peripheral blood mononuclear cells. Immunopharmacol. Immunotoxicol. 2012, 34, 686–694. [Google Scholar] [CrossRef]
- Gardiner, E.; Carroll, A.; Tooney, P.A.; Cairns, M.J. Antipsychotic drug-associated gene–miRNA interaction in T-lymphocytes. Int. J. Neuropsychopharmacol. 2014, 17, 929–943. [Google Scholar] [CrossRef] [Green Version]
- Divac, N.; Prostran, M.; Jakovcevski, I.; Cerovac, N. Second-Generation Antipsychotics and Extrapyramidal Adverse Effects. Biomed Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Aringhieri, S.; Carli, M.; Kolachalam, S.; Verdesca, V.; Cini, E.; Rossi, M.; McCormick, P.J.; Corsini, G.U.; Maggio, R.; Scarselli, M. Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacol. Ther. 2018, 192, 20–41. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y. What’s atypical about atypical antipsychotic drugs? Curr. Opin. Pharmacol. 2004, 4, 53–57. [Google Scholar] [CrossRef]
- Kuroki, T.; Nagao, N.; Nakahara, T. Neuropharmacology of second-generation antipsychotic drugs: a validity of the serotonin–dopamine hypothesis. Prog. Brain Res. 2008, 172, 199–212. [Google Scholar]
- Meltzer, H.; Massey, B. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr. Opin. Pharmacol. 2011, 11, 59–67. [Google Scholar] [CrossRef]
- Iken, K.; Chheng, S.; Fargin, A.; Goulet, A.-C.; Kouassi, E. Serotonin Upregulates Mitogen-Stimulated B Lymphocyte Proliferation through 5-HT1AReceptors. Cell. Immunol. 1995, 163, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Chiaravalli, A.M.; Mian, M.; Zucca, E.; Tibiletti, M.G.; Capella, C.; Bertoni, F. Serotonin Receptor 3A Expression in Normal and Neoplastic B Cells. Pathobiology 2010, 77, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Serafeim, A.; Grafton, G.; Chamba, A.; Gregory, C.D.; Blakely, R.D.; Bowery, N.G.; Barnes, N.M.; Gordon, J. 5-Hydroxytryptamine drives apoptosis in biopsylike Burkitt lymphoma cells: Reversal by selective serotonin reuptake inhibitors. Blood 2002, 99, 2545–2553. [Google Scholar] [CrossRef]
- Gorres, K.L.; Daigle, D.; Mohanram, S.; Miller, G. Activation and Repression of Epstein-Barr Virus and Kaposi’s Sarcoma-Associated Herpesvirus Lytic Cycles by Short- and Medium-Chain Fatty Acids. J. Virol. 2014, 88, 8028–8044. [Google Scholar] [CrossRef]
- Monti, B.; Polazzi, E.; Contestabile, A. Biochemical, Molecular and Epigenetic Mechanisms of Valproic Acid Neuroprotection. Curr. Mol. Pharmacol. 2010, 2, 95–109. [Google Scholar] [CrossRef]
- Medigeshi, G.R.; Kumar, R.; Dhamija, E.; Agrawal, T.; Kar, M. N-Desmethylclozapine, Fluoxetine, and Salmeterol Inhibit Postentry Stages of the Dengue Virus Life Cycle. Antimicrob. Agents Chemother. 2016, 60, 6709–6718. [Google Scholar] [CrossRef] [Green Version]
- Jones-Brando, L.V.; Buthod, J.L.; Holland, L.E.; Yolken, R.H.; Fuller Torrey, E. Metabolites of the antipsychotic agent clozapine inhibit the replication of human immunodeficiency virus type 1. Schizophr. Res. 1997, 25, 63–70. [Google Scholar] [CrossRef]
- Baum, S.; Ashok, A.; Gee, G.; Dimitrova, S.; Querbes, W.; Jordan, J.; Atwood, W.J. Early events in the life cycle of JC virus as potential therapeutic targets for the treatment of progressive multifocal leukoencephalopathy. J. Neurovirol. 2003, 9, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Diem, O.; Schäffner, M.; Seifarth, W.; Leib-Mösch, C. Influence of Antipsychotic Drugs on Human Endogenous Retrovirus (HERV) Transcription in Brain Cells. PLoS ONE 2012, 7, e30054. [Google Scholar] [CrossRef] [PubMed]
- Ornaghi, S.; Davis, J.N.; Gorres, K.L.; Miller, G.; Paidas, M.J.; van den Pol, A.N. Mood stabilizers inhibit cytomegalovirus infection. Virology 2016, 499, 121–135. [Google Scholar] [CrossRef] [Green Version]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, A.G.; Gaffy, C.B.; Weseli, J.R.; Gorres, K.L. Inhibition of Epstein-Barr Virus Lytic Reactivation by the Atypical Antipsychotic Drug Clozapine. Viruses 2019, 11, 450. https://doi.org/10.3390/v11050450
Anderson AG, Gaffy CB, Weseli JR, Gorres KL. Inhibition of Epstein-Barr Virus Lytic Reactivation by the Atypical Antipsychotic Drug Clozapine. Viruses. 2019; 11(5):450. https://doi.org/10.3390/v11050450
Chicago/Turabian StyleAnderson, Abbie G., Cullen B. Gaffy, Joshua R. Weseli, and Kelly L. Gorres. 2019. "Inhibition of Epstein-Barr Virus Lytic Reactivation by the Atypical Antipsychotic Drug Clozapine" Viruses 11, no. 5: 450. https://doi.org/10.3390/v11050450
APA StyleAnderson, A. G., Gaffy, C. B., Weseli, J. R., & Gorres, K. L. (2019). Inhibition of Epstein-Barr Virus Lytic Reactivation by the Atypical Antipsychotic Drug Clozapine. Viruses, 11(5), 450. https://doi.org/10.3390/v11050450