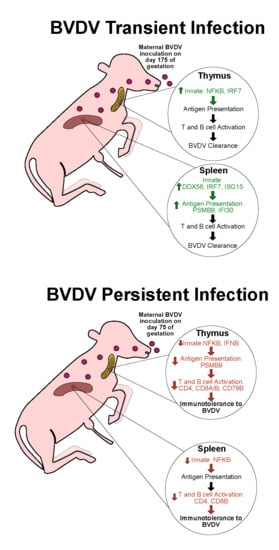
Fetal Lymphoid Organ Immune Responses to Transient and Persistent Infection with Bovine Viral Diarrhea Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design: BVDV Inoculation and Fetal Collections
2.3. RNA Extraction and RT-qPCR
2.4. RT-qPCR Targets and Validation
2.5. Morphogenesis of Thymus during Bovine Fetal Development and Effect of In Utero BVDV Infection
2.6. Statistical Analysis
3. Results
3.1. Detection of BVDV RNA Expression in Thymus and Spleen and BVDV Receptor CD46 in Fetal Thymuses
3.2. Thymic Responses
3.2.1. Innate Immune Responses in TI and PI Fetal Thymuses
3.2.2. Adaptive Immune Responses in TI and PI Fetal Thymuses
3.2.3. Morphogenesis and Histology of the Thymus during Bovine Fetal Development and the Effect of in Utero BVDV Infection
3.3. Splenic Responses
3.3.1. Innate Immune Responses in TI and PI Fetal Spleens
3.3.2. Adaptive Immune Responses in TI and PI Fetal Spleens
4. Discussion
4.1. Transient Infection on Day 175 of Gestation Elicits an Immunocompetent Response in the Bovine Fetal Thymus and Spleen on Day 190
4.2. PI Thymus and Spleen Exhibit Diminished or Inhibited Immune Responses at Day 190 of Gestation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Tissue | Target | Control Mean | Control SEM | TI Mean | TI SEM | PI Mean | PI SEM | TI v. C p value | PI v. C p value |
|---|---|---|---|---|---|---|---|---|---|
| Thymus | STAT1 | 1.32 | 0.48 | 2.29 | 0.21 | 0.04 | 0.01 | 0.58 | 0.07 |
| Thymus | IFI6 | 1.10 | 0.20 | 2.09 | 0.49 | 0.10 | 0.02 | 0.09 | 0.09 |
| Thymus | CXCL10 | 1.24 | 0.35 | 3.10 | 1.20 | 0.27 | 0.12 | 0.99 | 0.11 |
| Thymus | CXCL16 | 1.22 | 0.34 | 1.21 | 0.27 | 0.05 | 0.01 | 0.99 | 0.01 |
| Thymus | CXCR6 | 1.40 | 0.57 | 1.64 | 0.41 | 0.48 | 0.15 | 0.99 | 0.53 |
| Thymus | TAP1 | 1.09 | 0.20 | 1.07 | 0.21 | 0.20 | 0.06 | 0.99 | 0.01 |
| Thymus | B2M | 1.17 | 0.31 | 1.33 | 0.24 | 0.04 | 0.01 | 0.89 | 0.01 |
| Thymus | CIITA | 1.19 | 0.27 | 1.35 | 0.35 | 0.35 | 0.08 | 0.99 | 0.08 |
| Spleen | STAT4 | 1.62 | 0.83 | 2.25 | 0.51 | 0.32 | 0.04 | 0.43 | 0.08 |
| Spleen | PSMB8 | 1.66 | 0.79 | 6.09 | 1.40 | 0.06 | 0.02 | 0.21 | 0.07 |
References
- Moennig, V.; Houe, H.; Lindberg, A. BVD control in Europe: Current status and perspectives. Anim. Health Res. Rev. 2005, 6, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.; Pierce, V.; Grotelueschen, D.; Wittum, T. Economic evaluation of beef cowherd screening for cattle persistently-infected with bovine viral diarrhea virus. Bov. Pract. 2002, 36, 106–112. [Google Scholar]
- Pinior, B.; Firth, C.L.; Richter, V.; Bakran-Lebl, K.; Trauffler, M.; Dzieciol, M.; Hutter, S.E.; Burgstaller, J.; Obritzhauser, W.; Winter, P.; et al. A systematic review of financial and economic assessments of bovine viral diarrhea virus (BVDV) prevention and mitigation activities worldwide. Prev. Vet. Med. 2017, 137, 77–92. [Google Scholar] [CrossRef]
- Richter, V.; Bakran-Lebl, K.; Baumgartner, W.; Obritzhauser, W.; Käsbohrer, A.; Pinior, B. A systematic worldwide review of the direct monetary losses in cattle due to bovine viral diarrhoea virus infection. Vet. J. 2017, 220, 80–87. [Google Scholar] [CrossRef]
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV Virus Taxonomy Profile: Flaviviridae. J. Gen. Virol. 2017, 98, 2–3. [Google Scholar] [CrossRef]
- Tautz, N.; Tews, B.A.; Meyers, G. The Molecular Biology of Pestiviruses. Nat. Eng. Resist. Plant Viruses Part II 2015, 93, 47–160. [Google Scholar] [CrossRef]
- Bielefeldt-Ohmann, H. The Pathologies of Bovine Viral Diarrhea Virus Infection. Vet. Clin. N. Am. Food Anim. Pract. 1995, 11, 447–476. [Google Scholar] [CrossRef]
- Brownlie, J.; Clarke, M.C.; Howard, C.J.; Pocock, D.H. Pathogenesis and epidemiology of bovine virus diarrhoea virus infection of cattle. Ann. Rech. Vet. Ann. Vet. Res. 1987, 18, 157–166. [Google Scholar]
- Kendrick, J.W. Bovine viral diarrhea-mucosal disease virus infection in pregnant cows. Am. J. Vet. Res. 1971, 32, 533–544. [Google Scholar]
- Lanyon, S.; Hill, F.I.; Reichel, M.P.; Brownlie, J. Bovine viral diarrhoea: Pathogenesis and diagnosis. Vet. J. 2014, 199, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Olafson, P.; Maccallum, A.D.; Fox, F.H. An apparently new transmissible disease of cattle. Cornell Vet. 1946, 36, 205–213. [Google Scholar]
- Coria, M.F.; McClurkin, A.W. Duration of active and colostrum-derived passive antibodies to bovine viral diarrhea virus in calves. Can. J. Comp. Med. Rev. Can. Med. Comp. 1978, 42, 239–243. [Google Scholar]
- McClurkin, A.W.; Littledike, E.T.; Cutlip, R.C.; Frank, G.H.; Coria, M.F.; Bolin, S.R. Production of cattle immunotolerant to bovine viral diarrhea virus. Can. J. Comp. Med. Rev. Can. Med. Comp. 1984, 48, 156–161. [Google Scholar]
- Hansen, T.R.; Smirnova, N.P.; Webb, B.T.; Bielefeldt-Ohmann, H.; Sacco, R.E.; Van Campen, H. Innate and adaptive immune responses to in utero infection with bovine viral diarrhea virus. Anim. Health Res. Rev. 2015, 16, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, J.; Clarke, M.; Howard, C. Experimental production of fatal mucosal disease in cattle. Vet. Rec. 1984, 114, 535–536. [Google Scholar] [CrossRef]
- Van Campen, H. Bovine Viral Diarrhea. In Proceedings of the Range Beef Cow Symposium, Rapid City, SD, USA, 9–11 December 1997; University of Nebraska-Lincoln Commons: Rapid City, SD, USA, 1997. [Google Scholar]
- Potgieter, L.N. Immunology of Bovine Viral Diarrhea Virus. Vet. Clin. N. Am. Food Anim. Pract. 1995, 11, 501–520. [Google Scholar] [CrossRef]
- Schweizer, M.; Peterhans, E. Pestiviruses. Annu. Rev. Anim. Biosci. 2014, 2, 141–163. [Google Scholar] [CrossRef]
- Peterhans, E.; Schweizer, M. BVDV: A pestivirus inducing tolerance of the innate immune response. Biology 2013, 41, 39–51. [Google Scholar] [CrossRef]
- Baigent, S.J.; Goodbourn, S.; McCauley, J.W. Differential activation of interferon regulatory factors-3 and -7 by non-cytopathogenic and cytopathogenic bovine viral diarrhoea virus. Vet. Immunol. Immunopathol. 2004, 100, 135–144. [Google Scholar] [CrossRef]
- Baigent, S.J.; Zhang, G.; Fray, M.D.; Flick-Smith, H.; Goodbourn, S.; McCauley, J.W. Inhibition of Beta Interferon Transcription by Noncytopathogenic Bovine Viral Diarrhea Virus Is through an Interferon Regulatory Factor 3-Dependent Mechanism. J. Virol. 2002, 76, 8979–8988. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Rijnbrand, R.; Jangra, R.K.; Devaraj, S.G.; Qu, L.; Ma, Y.; Lemon, S.M.; Li, K. Ubiquitination and proteasomal degradation of interferon regulatory factor-3 induced by Npro from a cytopathic bovine viral diarrhea virus. Virology 2007, 366, 277–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, L.H.; Ansari, I.H.; Vassilev, V.; Liang, D.; Lai, V.C.; Zhong, W.; Hong, Z.; Dubovi, E.J.; Donis, R.O. The amino-terminal domain of bovine viral diarrhea virus Npro protein is necessary for alpha/beta interferon antagonism. J. Virol. 2006, 80, 900–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, L.H.V.G.; Van Olphen, A.L.; Mittal, S.K.; Donis, R.O. Modulation of PKR activity in cells infected by bovine viral diarrhea virus. Virus Res. 2006, 116, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Magouras, I.; Mätzener, P.; Rümenapf, T.; Peterhans, E.; Schweizer, M. RNase-dependent inhibition of extracellular, but not intracellular, dsRNA-induced interferon synthesis by Erns of pestiviruses. J. Gen. Virol. 2008, 89, 2501–2506. [Google Scholar] [CrossRef]
- Meyers, G.; Ege, A.; Fetzer, C.; Von Freyburg, M.; Elbers, K.; Carr, V.; Prentice, H.; Charleston, B.; Schürmann, E.-M. Bovine Viral Diarrhea Virus: Prevention of Persistent Fetal Infection by a Combination of Two Mutations Affecting Erns RNase and Npro Protease. J. Virol. 2007, 81, 3327–3338. [Google Scholar] [CrossRef] [Green Version]
- Charleston, B.; Fray, M.D.; Baigent, S.; Carr, B.V.; Morrison, W.I. Establishment of persistent infection with non-cytopathic bovine viral diarrhoea virus in cattle is associated with a failure to induce type I interferon. J. Gen. Virol. 2001, 82, 1893–1897. [Google Scholar] [CrossRef]
- Smirnova, N.P.; Webb, B.T.; Bielefeldt-Ohmann, H.; Van Campen, H.; Antoniazzi, A.Q.; Morarie, S.E.; Hansen, T.R. Development of fetal and placental innate immune responses during establishment of persistent infection with bovine viral diarrhea virus. Virus Res. 2012, 167, 329–336. [Google Scholar] [CrossRef]
- Smirnova, N.P.; Ptitsyn, A.; Austin, K.J.; Bielefeldt-Ohmann, H.; Van Campen, H.; Han, H.; Van Olphen, A.L.; Hansen, T.R. Persistent fetal infection with bovine viral diarrhea virus differentially affects maternal blood cell signal transduction pathways. Physiol. Genom. 2008, 36, 129–139. [Google Scholar] [CrossRef]
- Ohmann, H.B.; Rønsholt, L.; Bloch, B. Demonstration of Bovine Viral Diarrhoea Virus in Peripheral Blood Mononuclear Cells of Persistently Infected, Clinically Normal Cattle. J. Gen. Virol. 1987, 68, 1971–1982. [Google Scholar] [CrossRef]
- Shoemaker, M.L.; Smirnova, N.P.; Bielefeldt-Ohmann, H.; Austin, K.J.; Van Olphen, A.; Clapper, J.A.; Hansen, T.R. Differential Expression of the Type I Interferon Pathway during Persistent and Transient Bovine Viral Diarrhea Virus Infection. J. Interf. Cytokine Res. 2009, 29, 23–36. [Google Scholar] [CrossRef]
- Palomares, R.A.; Walz, H.G.; Brock, K.V. Expression of type I interferon-induced antiviral state and pro-apoptosis markers during experimental infection with low or high virulence bovine viral diarrhea virus in beef calves. Virus Res. 2013, 173, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Charleston, B.; Brackenbury, L.S.; Carr, B.V.; Fray, M.D.; Hope, J.C.; Howard, C.J.; Morrison, W.I. Alpha/Beta and Gamma Interferons are Induced by Infection with Noncytopathic Bovine Viral Diarrhea Virus In Vivo. J. Virol. 2002, 76, 923–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnova, N.P.; Webb, B.; McGill, J.L.; Schaut, R.G.; Bielefeldt-Ohmann, H.; Van Campen, H.; Sacco, R.E.; Hansen, T.R. Induction of interferon-gamma and downstream pathways during establishment of fetal persistent infection with bovine viral diarrhea virus. Virus Res. 2014, 183, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Georges, H.M.; Knapek, K.J.; Bielefeldt-Ohmann, H.; Van Campen, H.; Hansen, T.R. Attenuated lymphocyte activation leads to the development of immunotolerance in bovine fetuses persistently infected with BVDV†. Biol. Reprod. 2020. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.F.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Smirnova, N.P.; Bielefeldt-Ohmann, H.; Van Campen, H.; Austin, K.J.; Han, H.; Montgomery, D.L.; Shoemaker, M.L.; Van Olphen, A.L.; Hansen, T.R. Acute non-cytopathic bovine viral diarrhea virus infection induces pronounced type I interferon response in pregnant cows and fetuses. Virus Res. 2008, 132, 49–58. [Google Scholar] [CrossRef]
- Goddeeris, B.M.; Morrison, W.I. Cell-Mediated Immunity in Ruminants; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Schultz, R.D.; Dunne, H.W.; Heist, C.E. Ontogeny of the Bovine Immune Response 1. Infect. Immun. 1973, 7, 981–991. [Google Scholar] [CrossRef] [Green Version]
- Haynes, B.F. The Human Thymic Microenvironment. Adv. Immunol. 1984, 36, 87–142. [Google Scholar] [CrossRef]
- Lobach, D.F.; Haynes, B.F. Ontogeny of the human thymus during fetal development. J. Clin. Immunol. 1987, 7, 81–97. [Google Scholar] [CrossRef]
- Ohmann, H.B. Experimental fetal infection with bovine viral diarrhea virus. II. Morphological reactions and distribution of viral antigen. Can. J. Comp. Med. Rev. Can. Med. Comp. 1982, 46, 363–369. [Google Scholar]
- Done, J.; Terlecki, S.; Richardson, C.; Harkness, J.; Sands, J.; Patterson, D.; Sweasey, D.; Shaw, I.; Winkler, C.; Duffell, S. Bovine virus diarrhoea-mucosal disease virus: Pathogenicity for the fetal calf following maternal infection. Vet. Rec. 1980, 106, 473–479. [Google Scholar] [CrossRef]
- Falkenberg, S.M.; Bauermann, F.V.; Ridpath, J.F. Characterization of thymus-associated lymphoid depletion in bovine calves acutely or persistently infected with bovine viral diarrhea virus 1, bovine viral diarrhea virus 2 or HoBi-like pestivirus. Arch. Virol. 2017, 162, 3473–3480. [Google Scholar] [CrossRef] [PubMed]
- Golub, R.; Tan, J.; Watanabe, T.; Brendolan, A. Origin and Immunological Functions of Spleen Stromal Cells. Trends Immunol. 2018, 39, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Davis, W.C.; Belden, E.L.; Pratt, D.L. Flow Cytofluorimetric Analysis of Lymphocyte Subset Alterations in Cattle Infected with Bovine Viral Diarrhea Virus. Vet. Pathol. 1988, 25, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Bolin, S.R.; McClurkin, A.W.; Coria, M.F. Effects of bovine viral diarrhea virus on the percentages and absolute num- bers of circulating B and T lymphocytes in cattle. Am. J. Vet. Res. 1988, 46, 884–886. [Google Scholar]
- Burt, T.D. Fetal regulatory T cells and peripheral immune tolerance in utero: Implications for development and disease. Am. J. Reprod. Immunol. 2013, 69, 346–358. [Google Scholar] [CrossRef] [Green Version]
- Mold, J.E.; Venkatasubrahmanyam, S.; Burt, T.D.; Michaëlsson, J.; Rivera, J.M.; Galkina, S.A.; Weinberg, K.; Stoddart, C.A.; McCune, J.M. Fetal and Adult Hematopoietic Stem Cells Give Rise to Distinct T Cell Lineages in Humans. Science 2010, 330, 1695–1699. [Google Scholar] [CrossRef] [Green Version]
- Collen, T.; Douglas, A.J.; Paton, D.J.; Zhang, G.; Morrison, W. Single Amino Acid Differences are Sufficient for CD4+ T-Cell Recognition of a Heterologous Virus by Cattle Persistently Infected with Bovine Viral Diarrhea Virus. Virology 2000, 276, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Fu, Y. The complicated role of NF-kappaB in T-cell selection. Cell Mol. Immunol. 2010, 7, 89–93. [Google Scholar] [CrossRef]
- Leonidas, C.P. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar]
- Thompson, M.R.; Sharma, S.; Atianand, M.; Jensen, S.B.; Carpenter, S.; Knipe, D.M.; Fitzgerald, K.A.; Kurt-Jones, E.A. Interferon γ-inducible Protein (IFI) 16 Transcriptionally Regulates Type I Interferons and Other Interferon-stimulated Genes and Controls the Interferon Response to both DNA and RNA Viruses*. J. Biol. Chem. 2014, 289, 23568–23581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harwig, A.; Landick, R.; Berkhout, B. The Battle of RNA Synthesis: Virus versus Host. Viruses 2017, 9, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedictus, L.; Luteijn, R.D.; Otten, H.; Lebbink, R.J.; Van Kooten, P.J.S.; Wiertz, E.J.H.J.; Rutten, V.P.M.G.; Koets, A. Pathogenicity of Bovine Neonatal Pancytopenia-associated vaccine-induced alloantibodies correlates with Major Histocompatibility Complex class I expression. Sci. Rep. 2015, 5, 12748. [Google Scholar] [CrossRef] [Green Version]
- Cyster, J.G. Chemokines and Cell Migration in Secondary Lymphoid Organs. Science 1999, 286, 2098–2102. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.A.; Haxhinasto, S.A.; Stunz, L.L.; Hostager, B.S. Antigen-specific B-lymphocyte activation. Crit. Rev. Immunol. 2003, 23, 149–197. [Google Scholar] [CrossRef]
- Maurer, K.; Krey, T.; Moennig, V.; Thiel, H.-J.; Rümenapf, T. CD46 Is a Cellular Receptor for Bovine Viral Diarrhea Virus. J. Virol. 2004, 78, 1792–1799. [Google Scholar] [CrossRef] [Green Version]
- Krey, T.; Himmelreich, A.; Heimann, M.; Menge, C.; Thiel, H.-J.; Maurer, K.; Rümenapf, T. Function of Bovine CD46 as a Cellular Receptor for Bovine Viral Diarrhea Virus Is Determined by Complement Control Protein 1. J. Virol. 2006, 80, 3912–3922. [Google Scholar] [CrossRef] [Green Version]
- Neill, J.D.; Ridpath, J.F. Gene expression changes in BVDV2-infected MDBK cells. Biology 2003, 31, 97–102. [Google Scholar] [CrossRef]
- Alzamel, N.; Bayrou, C.; Decreux, A.; Desmecht, D. Soluble forms of CD46 are detected in Bos taurus plasma and neutralize BVDV, the bovine pestivirus. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 39–46. [Google Scholar] [CrossRef]






| Gene | Sequence | Accession | Efficiency |
|---|---|---|---|
| 18S | FW: GAACGAGACTCTGGGCATGC | NR_036642 | 102% |
| REV: CTGAACGCCACTTGTCCCTC | |||
| DDX58 | FW: GAGCACTGGTGGATGCCTTA | XM_024996055.1 | 157% |
| REV: GCTGTCTCTGTTGGTTCGGA | |||
| IRF7 | FW: GCCTCCTGGAAAACCAACTT | NM_001105040.1 | 127% |
| REV: CCTTATGAGGGTCGGTAGGGG | |||
| NFKB | FW: CGAGGTTCGGTTCTACGAGG | NM_001102101.1 | 134% |
| REV: TGCAGGAACACGGGTTACAGG | |||
| IFNB | FW: TCCAGCACATCTTCGGCATT | NM_174350.1 | 104% |
| REV: TTCCCTAGGTGGGGAACGAT | |||
| ISG15 | FW: GGTATCCGAGCTGAAGCAGTT | NM_174366 | 115% |
| REV: ACCTCCCTGCTGTCAAGGT | |||
| STAT4 | FW: TTCTTCCCATGTCGCCAAGT | NM_001083692.2 | 104% |
| REV: AACCAGATGTGATTGTTGGCA | |||
| IFI30 | FW: GCATGCAGCTCTTGCACATC | NM_001101251.2 | 131% |
| REV: GGCCCCAAGAGTTCTTACCC | |||
| PSMB9 | FW: ATCTACCTGGCCACCATCAC | NM_001034388 | 115% |
| REV: AGGAGAGTCCGAGGAAGGAG | |||
| PSMB8 | FW: ACTGGAAGGCAGCACAGAGT | NM_001040480 | 124% |
| REV: ATTGTGCTTAGTGGGGCATC | |||
| B2M | FW: AFTAAGCCGCAGTGGAGGT | AC_000167.1 | 123% |
| Rev: CGCAAAACACCCTGAAGACT | |||
| CXCL10 | FW: ACACCGAGGCACTACGTTCT | CB533091 | 121% |
| Rev: TAAGCCCAGAGCTGGAAAGA | |||
| CXCL16 | FW: CTTGTGAGGGCAGATTGTGA | CK770974 | 105% |
| Rev:GGTCAATAGCTGGTTAGTTGTGAA | |||
| CD4 | FW: GGGCAGAACGGATGTCTCAA | NM_001103225.1 | 110% |
| REV: ATAGGTCTTCTGGAGCCGGT | |||
| CD8a | FW: TACATCTGGGCTCCCTTGGT | NM_174015.1 | 130% |
| REV: CCACAGGCCTGGGACATTTG | |||
| CD8b | FW: AGCTGAGTGTGTTGATGTTCT | NM_001105344.2 | 93% |
| REV: TTCTGAGTCACCTGGGTTGG | |||
| CD79b | FW: TGATTCCCGGGCTCAACAAC | XM_002696068.6 | 160% |
| REV: CTGCCAGATCCGGGAACAAG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knapek, K.J.; Georges, H.M.; Van Campen, H.; Bishop, J.V.; Bielefeldt-Ohmann, H.; Smirnova, N.P.; Hansen, T.R. Fetal Lymphoid Organ Immune Responses to Transient and Persistent Infection with Bovine Viral Diarrhea Virus. Viruses 2020, 12, 816. https://doi.org/10.3390/v12080816
Knapek KJ, Georges HM, Van Campen H, Bishop JV, Bielefeldt-Ohmann H, Smirnova NP, Hansen TR. Fetal Lymphoid Organ Immune Responses to Transient and Persistent Infection with Bovine Viral Diarrhea Virus. Viruses. 2020; 12(8):816. https://doi.org/10.3390/v12080816
Chicago/Turabian StyleKnapek, Katie J., Hanah M. Georges, Hana Van Campen, Jeanette V. Bishop, Helle Bielefeldt-Ohmann, Natalia P. Smirnova, and Thomas R. Hansen. 2020. "Fetal Lymphoid Organ Immune Responses to Transient and Persistent Infection with Bovine Viral Diarrhea Virus" Viruses 12, no. 8: 816. https://doi.org/10.3390/v12080816
APA StyleKnapek, K. J., Georges, H. M., Van Campen, H., Bishop, J. V., Bielefeldt-Ohmann, H., Smirnova, N. P., & Hansen, T. R. (2020). Fetal Lymphoid Organ Immune Responses to Transient and Persistent Infection with Bovine Viral Diarrhea Virus. Viruses, 12(8), 816. https://doi.org/10.3390/v12080816