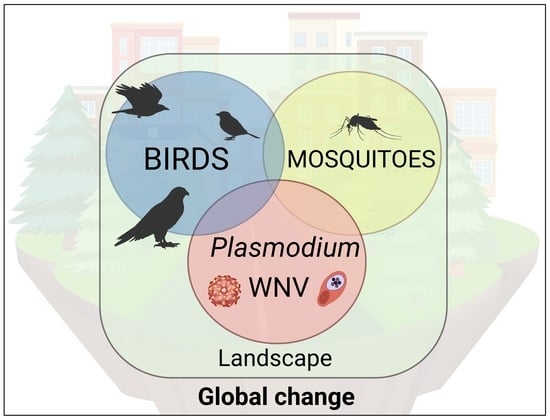
Ecological Effects on the Dynamics of West Nile Virus and Avian Plasmodium: The Importance of Mosquito Communities and Landscape
Abstract
:1. Introduction
1.1. A General Perspective of Vector-Borne Diseases
1.2. West Nile Virus and Avian Plasmodium Parasites
2. Effects of Landscape Change on West Nile Virus and Avian Plasmodium
3. Pathogen Prevalence and Mosquito Community Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Vector-Borne Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 12 March 2021).
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Trends in reported vectorborne disease cases—United States and territories, 2004–2016. Morb. Mortal. Wkly. Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patz, J.A.; Graczyk, T.K.; Geller, N.; Vittor, A.Y. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 2000, 30, 1395–1405. [Google Scholar] [CrossRef] [Green Version]
- Kilpatrick, A.M.; Randolph, S.E. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 2012, 380, 1946–1955. [Google Scholar] [CrossRef] [Green Version]
- Wiethoelter, A.K.; Beltrán-Alcrudo, D.; Kock, R.; Mor, S.M. Global trends in infectious diseases at the wildlife-livestock interface. Proc. Natl. Acad. Sci. USA 2015, 112, 9662–9667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorou, R.M.; Papavassiliou, V.G.; Tsiodras, S. Emerging zoonoses and vector-borne infections affecting humans in Europe. Epidemiol. Infect. 2007, 135, 1231–1247. [Google Scholar] [CrossRef] [PubMed]
- Franklinos, L.H.V.; Jones, K.E.; Redding, D.W.; Abubakar, I. The effect of global change on mosquito-borne disease. Lancet Infect. Dis. 2019, 19, e302–e312. [Google Scholar] [CrossRef]
- Combes, C. Parasitism: The Ecology and Evolution of Intimate Iinteractions; University of Chicago Press: Chicago, IL, USA, 2001. [Google Scholar]
- Campbell-Lendrum, D.; Molyneux, D.; Amerasinghe, F.; Davies, C.; Fletcher, E.; Schofield, C.; Hougard, J.-M.; Polson, K.; Sinkins, S.; Epstein, P.; et al. Ecosystems and vector-borne disease control. In Ecosystems and Human Well–Being: Policy Responses; Epstein, P., Githeko, A., Rabinovich, J., Weinstein, P., Eds.; Editorial Island: Washington, DC, USA, 2005; pp. 353–372. [Google Scholar]
- Sehgal, R.N.M. Manifold habitat effects on the prevalence and diversity of avian blood parasites. Int. J. Parasitol. Parasites Wildl. 2015, 4, 421–430. [Google Scholar] [CrossRef] [Green Version]
- Zeller, H.G.; Schuffenecker, I. West Nile virus: An overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef]
- Rizzoli, A.; Jiménez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The challenge of West Nile virus in Europe: Knowledge gaps and research priorities. Eurosurveillance 2015, 20, 21135. [Google Scholar] [CrossRef] [Green Version]
- Jupp, P.G. The ecology of West Nile virus in South Africa and the occurrence of outbreaks in humans. Ann. N. Y. Acad. Sci. 2001, 951, 143–152. [Google Scholar] [CrossRef]
- Reisen, W.K.; Hayes, C.G.; Azra, K.; Niaz, S.; Mahmood, F.; Parveen, T.; Boreham, P.F.L. West Nile virus in Pakistan. II. Entomological studies at Changa Manga National Forest, Punjab Province. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 437–448. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Roehrig, J.T.; Deubel, V.; Smith, J.; Parker, M.; Steele, K.; Crise, B.; Volpe, K.E.; Crabtree, M.B.; Scherret, J.H.; et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the Northeastern United States. Science 1999, 286, 2333–2337. [Google Scholar] [CrossRef] [Green Version]
- Ciccozzi, M.; Peletto, S.; Cella, E.; Giovanetti, M.; Lai, A.; Gabanelli, E.; Acutis, P.L.; Modesto, P.; Rezza, G.; Platonov, A.E.; et al. Epidemiological history and phylogeography of West Nile virus lineage 2. Infect. Genet. Evol. 2013, 17, 46–50. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ivanics, É.; Erdélyi, K.; Ursu, K.; Ferenczi, E.; Weissenböck, H.; Nowotny, N. Lineage 1 and 2 strains of encephalitic West Nile virus, Central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef]
- Busquets, N.; Laranjo-González, M.; Soler, M.; Nicolás, O.; Rivas, R.; Talavera, S.; Villalba, R.; San Miguel, E.; Torner, N.; Aranda, C.; et al. Detection of West Nile virus lineage 2 in North-Eastern Spain (Catalonia). Transbound. Emerg. Dis. 2018, 66, 617–621. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, A.; Sánchez-Seco, M.P.; Ruiz, S.; Molero, F.; Hernández, L.; Moreno, J.; Magallanes, A.; Tejedor, C.G.; Tenorio, A. Putative new lineage of West Nile virus, Spain. Emerg. Infect. Dis. 2010, 16, 549–552. [Google Scholar] [CrossRef]
- Lvov, D.K.; Butenko, A.M.; Gromashevsky, V.L.; Kovtunov, A.I.; Prilipov, A.G.; Kinney, R.; Aristova, V.A.; Dzharkenov, A.F.; Samokhvalov, E.I.; Savage, H.M.; et al. West Nile virus and other zoonotic viruses in Russia: Examples of emerging-reemerging situations. In Emergence and Control of Zoonotic Viral Encephalitides; Springer: Vienna, Austria, 2004; pp. 85–96. [Google Scholar]
- Engler, O.; Savini, G.; Papa, A.; Figuerola, J.; Groschup, M.H.; Kampen, H.; Medlock, J.; Vaux, A.; Wilson, A.J.; Werner, D.; et al. European surveillance for West Nile virus in mosquito populations. Int. J. Environ. Res. Public Health 2013, 10, 4869–4895. [Google Scholar] [CrossRef] [Green Version]
- Van der Meulen, K.M.; Pensaert, M.B.; Nauwynck, H.J. West Nile virus in the vertebrate world. Arch. Virol. 2005, 150, 637–657. [Google Scholar] [CrossRef]
- Pérez-Ramírez, E.; Llorente, F.; Jiménez-Clavero, M.Á. Experimental infections of wild birds with West Nile virus. Viruses 2014, 6, 752–781. [Google Scholar] [CrossRef] [Green Version]
- Marra, P.P.; Griffing, S.; Caffrey, C.; Kilpatrick, M.A.; McLean, R.; Brand, C.; Saito, E.; Dupuis, A.P.; Kramer, L.; Novak, R. West Nile virus and wildlife. Bioscience 2004, 54, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Rizzoli, A.; Bolzoni, L.; Chadwick, E.A.; Capelli, G.; Montarsi, F.; Grisenti, M.; Martínez-de la Puente, J.; Muñoz, J.; Figuerola, J.; Soriguer, R.; et al. Understanding West Nile virus ecology in Europe: Culex pipiens host feeding preference in a hotspot of virus emergence. Parasit. Vectors 2015, 8, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, R.G.; Ubico, S.R.; Bourne, D.; Komar, N. West Nile virus in livestock and wildlife. Curr. Top. Microbiol. Immunol. 2002, 267, 271–308. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, A.M.; Daszak, P.; Jones, M.J.; Marra, P.P.; Kramer, L.D. Host heterogeneity dominates West Nile virus transmission. Proc. R. Soc. B Biol. Sci. 2006, 273, 2327–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malkinson, M.; Banet, C. The role of birds in the ecology of West Nile virus in Europe and Africa. Curr. Top. Microbiol. Immunol. 2002, 267, 309–322. [Google Scholar] [CrossRef]
- Figuerola, J.; Angel Jiménez-Clavero, M.; López, G.; Rubio, C.; Soriguer, R.; Gómez-Tejedor, C.; Tenorio, A.; Jiménez-Clavero, M.A.; López, G.; Rubio, C.; et al. Size matters: West Nile Virus neutralizing antibodies in resident and migratory birds in Spain. Vet. Microbiol. 2008, 132, 39–46. [Google Scholar] [CrossRef]
- Höfle, U.; Blanco, J.M.; Crespo, E.; Naranjo, V.; Jiménez-Clavero, M.A.; Sanchez, A.; de la Fuente, J.; Gortazar, C. West Nile virus in the endangered Spanish imperial eagle. Vet. Microbiol. 2008, 129, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.M.; Brault, A.C.; van Amerongen, G.; Bosco-Lauth, A.M.; Romo, H.; Sewbalaksing, V.D.; Bowen, R.A.; Osterhaus, A.D.M.E.; Koraka, P.; Martina, B.E.E. Susceptibility of carrion crows to experimental infection with lineage 1 and 2 West Nile viruses. Emerg. Infect. Dis. 2015, 21, 1357–1365. [Google Scholar] [CrossRef]
- Vidaña, B.; Busquets, N.; Napp, S.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Johnson, N. The role of birds of prey in West Nile virus epidemiology. Vaccines 2020, 8, 550. [Google Scholar] [CrossRef]
- Feyer, S.; Bartenschlager, F.; Bertram, C.A.; Ziegler, U.; Fast, C.; Klopfleisch, R.; Müller, K. Clinical, pathological and virological aspects of fatal West Nile virus infections in ten free-ranging goshawks (Accipiter gentilis) in Germany. Transbound. Emerg. Dis. 2021, 68, 907–919. [Google Scholar] [CrossRef]
- Wodak, E.; Richter, S.; Bagó, Z.; Revilla-Fernández, S.; Weissenböck, H.; Nowotny, N.; Winter, P. Detection and molecular analysis of West Nile virus infections in birds of prey in the eastern part of Austria in 2008 and 2009. Vet. Microbiol. 2011, 149, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Erdélyi, K.; Ursu, K.; Ferenczi, E.; Szeredi, L.; Rátz, F.; Skáre, J.; Bakonyi, T. Clinical and pathologic features of lineage 2 West Nile virus infections in birds of prey in Hungary. Vector Borne Zoonotic Dis. 2007, 7, 181–188. [Google Scholar] [CrossRef]
- Gyure, K.A. West Nile Virus Infections. J. Neuropathol. Exp. Neurol. 2009, 68, 1053–1060. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Olivares, J.; Wood, J. West Nile virus infection of horses. Vet. Res. 2004, 35, 467–483. [Google Scholar] [CrossRef] [Green Version]
- Gossner, C.M.; Marrama, L.; Carson, M.; Allerberger, F.; Calistri, P.; Dilaveris, D.; Lecollinet, S.; Morgan, D.; Nowotny, N.; Paty, M.-C.; et al. West Nile virus surveillance in Europe: Moving towards an integrated animal-human-vector approach. Eurosurveillance 2017, 22, 30526. [Google Scholar] [CrossRef]
- ECDC. Epidemiological Update: West Nile Virus Transmission Season in Europe. 2018. Available online: https://www.ecdc.europa.eu/en/news-events/epidemiological-update-west-nile-virus-transmission-season-europe-2018 (accessed on 23 February 2021).
- Rodríguez-Alarcón, L.G.S.M.; Fernández-Martínez, B.; Sierra Moros, M.J.; Vázquez, A.; Julián Pachés, P.; García Villacieros, E.; Gómez Martín, M.B.; Figuerola Borras, J.; Lorusso, N.; Ramos Aceitero, J.M.; et al. Unprecedented increase of West Nile virus neuroinvasive disease, Spain, summer 2020. Eurosurveillance 2021, 26, 2002010. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Williams, R.B. Avian malaria: Clinical and chemical pathology of Plasmodium gallinaceum in the domesticated fowl Gallus gallus. Avian Pathol. 2005, 34, 29–47. [Google Scholar] [CrossRef]
- Bueno, M.G.; Lopez, R.P.G.; de Menezes, R.M.T.; de Costa-Nascimento, M.J.; de Lima, G.F.M.C.; de Araújo, R.A.S.; Guida, F.J.V.; Kirchgatter, K. Identification of Plasmodium relictum causing mortality in penguins (Spheniscus magellanicus) from São Paulo Zoo, Brazil. Vet. Parasitol. 2010, 173, 123–127. [Google Scholar] [CrossRef]
- Atkinson, C.T.; Samuel, M.D. Avian malaria Plasmodium relictum in native Hawaiian forest birds: Epizootiology and demographic impacts on ‘apapane Himatione sanguinea. J. Avian Biol. 2010, 41, 357–366. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Santiago-Alarcon, D.; Palinauskas, V.; Bensch, S. Plasmodium relictum. Trends Parasitol. 2021, 37, 355–356. [Google Scholar] [CrossRef]
- Asghar, M.; Hasselquist, D.; Hansson, B.; Zehtindjiev, P.; Westerdahl, H.; Bensch, S. Hidden costs of infection: Chronic malaria accelerates telomere degradation and senescence in wild birds. Science 2015, 347, 436–438. [Google Scholar] [CrossRef]
- Marzal, A.; Ricklefs, R.E.; Valkiunas, G.; Albayrak, T.; Arriero, E.; Bonneaud, C.; Czirják, G.A.; Ewen, J.; Hellgren, O.; Hořáková, D.; et al. Diversity, loss, and gain of malaria parasites in a globally invasive bird. PLoS ONE 2011, 6, e21905. [Google Scholar] [CrossRef] [Green Version]
- Cellier-Holzem, E.; Esparza-Salas, R.; Garnier, S.; Sorci, G. Effect of repeated exposure to Plasmodium relictum (lineage SGS1) on infection dynamics in domestic canaries. Int. J. Parasitol. 2010, 40, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E.; Fallon, S.M.; Bermingham, E. Evolutionary relationships, cospeciation, and host switching in avian malaria parasites. Syst. Biol. 2004, 53, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Moreno, J.; Sanz, J.J.; Arriero, E. Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc. R. Soc. B Biol. Sci. 2000, 267, 2507–2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinsen, E.S.; Perkins, S.L.; Schall, J.J. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): Evolution of life-history traits and host switches. Mol. Phylogenet. Evol. 2008, 47, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Lachish, S.; Knowles, S.C.L.; Alves, R.; Wood, M.J.; Sheldon, B.C. Infection dynamics of endemic malaria in a wild bird population: Parasite species-dependent drivers of spatial and temporal variation in transmission rates. J. Anim. Ecol. 2011, 80, 1207–1216. [Google Scholar] [CrossRef] [Green Version]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- Sotelo, E.; Llorente, F.; Rebollo, B.; Camuñas, A.; Venteo, A.; Gallardo, C.; Lubisi, A.; Rodríguez, M.J.; Sanz, A.J.; Figuerola, J.; et al. Development and evaluation of a new epitope-blocking ELISA for universal detection of antibodies to West Nile virus. J. Virol. Methods 2011, 174, 35–41. [Google Scholar] [CrossRef]
- Llorente, F.; García-Irazábal, A.; Pérez-Ramírez, E.; Cano-Gómez, C.; Sarasa, M.; Vázquez, A.; Jiménez-Clavero, M.Á. Influence of flavivirus co-circulation in serological diagnostics and surveillance: A model of study using West Nile, Usutu and Bagaza viruses. Transbound. Emerg. Dis. 2019, 66, 2100–2106. [Google Scholar] [CrossRef]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrhial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Cranfield, M.R.; Shiff, C.J. ELISA method for detecting anti-Plasmodium relictum and anti-Plasmodium elongatum antibody in infected duckling sera using Plasmodium falciparum antigens. J. Parasitol. 1993, 79, 879–885. [Google Scholar] [CrossRef]
- Atkinson, C.T.; Dusek, R.J.; Lease, J.K. Serological responses and immunity to superinfection with avian malaria in experimentally-infected Hawaii Amakihi. J. Wildl. Dis. 2001, 37, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, J.; Ruiz, S.; Soriguer, R.; Alcaide, M.; Viana, D.S.; Roiz, D.; Vázquez, A.; Figuerola, J. Feeding patterns of potential West Nile virus vectors in South-West Spain. PLoS ONE 2012, 7, e39549. [Google Scholar] [CrossRef] [Green Version]
- Balenghien, T.; Vazeille, M.; Grandadam, M.; Schaffner, F.; Zeller, H.; Reiter, P.; Sabatier, P.; Fouque, F.; Bicout, D.J. Vector competence of some French Culex and Aedes mosquitoes for West Nile virus. Vector Borne Zoonotic Dis. 2008, 8, 589–595. [Google Scholar] [CrossRef]
- Ergunay, K.; Gunay, F.; Oter, K.; Kasap, O.E.; Orsten, S.; Akkutay, A.Z.; Erdem, H.; Ozkul, A.; Alten, B. Arboviral surveillance of field-collected mosquitoes reveals circulation of west Nile virus lineage 1 strains in Eastern Thrace, Turkey. Vector Borne Zoonotic Dis. 2013, 13, 744–752. [Google Scholar] [CrossRef]
- Kimura, M.; Darbro, J.M.; Harrington, L.C. Avian malaria parasites share congeneric mosquito vectors. J. Parasitol. 2010, 96, 144–151. [Google Scholar] [CrossRef]
- Santiago-Alarcon, D.; Palinauskas, V.; Schaefer, H.M. Diptera vectors of avian Haemosporidian parasites: Untangling parasite life cycles and their taxonomy. Biol. Rev. 2012, 87, 928–964. [Google Scholar] [CrossRef]
- Ferraguti, M.; Heesterbeek, H.; Martínez-de la Puente, J.; Jiménez-Clavero, M.Á.; Vázquez, A.; Ruiz, S.; Llorente, F.; Roiz, D.; Vernooij, H.; Soriguer, R.; et al. The role of different Culex mosquito species in the transmission of West Nile virus and avian malaria parasites in Mediterranean areas. Transbound. Emerg. Dis. 2021, 68, 920–930. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de la Puente, J.; Muñoz, J.; Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Avian Pasmodium in Culex and Ochlerotatus mosquitoes from southern Spain: Effects of season and host-feeding source on parasite dynamics. PLoS ONE 2013, 8, e66237. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, C.; Martínez-de la Puente, J.; Figuerola, J.; Pérez-Cutillas, P.; Navarro, R.; Ortuño, M.; Bernal, L.J.; Ortiz, J.; Soriguer, R.; Berriatua, E. Molecular xenomonitoring and host identification of Leishmania sand fly vectors in a Mediterranean periurban wildlife park. Transbound. Emerg. Dis. 2019, 66, 2546–2561. [Google Scholar] [CrossRef] [PubMed]
- Latrofa, M.S.; Montarsi, F.; Ciocchetta, S.; Annoscia, G.; Dantas-Torres, F.; Ravagnan, S.; Capelli, G.; Otranto, D. Molecular xenomonitoring of Dirofilaria immitis and Dirofilaria repens in mosquitoes from north-eastern Italy by real-time PCR coupled with melting curve analysis. Parasit. Vectors 2012, 5, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valkiūnas, G. Haemosporidian vector research: Marriage of molecular and microscopical approaches is essential. Mol. Ecol. 2011, 20, 3084–3086. [Google Scholar] [CrossRef]
- Gutiérrez-López, R.; Martínez-de la Puente, J.; Gangoso, L.; Soriguer, R.; Figuerola, J. Plasmodium transmission differs between mosquito species and parasite lineages. Parasitology 2020, 147, 441–447. [Google Scholar] [CrossRef]
- Medeiros, M.C.I.; Anderson, T.K.; Higashiguchi, J.M.; Kitron, U.D.; Walker, E.D.; Brawn, J.D.; Krebs, B.L.; Ruiz, M.O.; Goldberg, T.L.; Ricklefs, R.E.; et al. An inverse association between West Nile virus serostatus and avian malaria infection status. Parasit. Vectors 2014, 7, 415. [Google Scholar] [CrossRef] [Green Version]
- Rijks, J.; Kik, M.; Slaterus, R.; Foppen, R.; Stroo, A.; IJzer, J.; Stahl, J.; Gröne, A.; Koopmans, M.; van der Jeugd, H.; et al. Widespread Usutu virus outbreak in birds in the Netherlands, 2016. Eurosurveillance 2016, 21, 30391. [Google Scholar] [CrossRef]
- Rouffaer, L.O.; Steensels, M.; Verlinden, M.; Vervaeke, M.; Boonyarittichaikij, R.; Martel, A.; Lambrecht, B. Usutu virus epizootic and Plasmodium coinfection in Eurasian Blackbirds (Turdus merula) in Flanders, Belgium. J. Wildl. Dis. 2018, 54, 859–862. [Google Scholar] [CrossRef]
- Marzal, A.; Bensch, S.; Reviriego, M.; Balbontin, J.; De Lope, F. Effects of malaria double infection in birds: One plus one is not two. J. Evol. Biol. 2008, 21, 979–987. [Google Scholar] [CrossRef]
- Medeiros, M.C.I.; Ricklefs, R.E.; Brawn, J.D.; Ruiz, M.O.; Goldberg, T.L.; Hamer, G.L. Overlap in the seasonal infection patterns of avian malaria parasites and West Nile virus in vectors and hosts. Am. J. Trop. Med. Hyg. 2016, 95, 1121–1129. [Google Scholar] [CrossRef]
- Roche, B.; Rohani, P.; Dobson, A.P.; Guégan, J.F. The impact of community organization on vector-borne pathogens. Am. Nat. 2013, 181, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shocket, M.S.; Verwillow, A.B.; Numazu, M.G.; Slamani, H.; Cohen, J.M.; El Moustaid, F.; Rohr, J.; Johnson, L.R.; Mordecai, E.A. Transmission of West Nile and five other temperate mosquito-borne viruses peaks at temperatures between 23 °C and 26 °C. eLife 2020, 9, e58511. [Google Scholar] [CrossRef]
- Folly, A.J.; Dorey-Robinson, D.; Hernández-Triana, L.M.; Ackroyd, S.; Vidana, B.; Lean, F.Z.X.; Hicks, D.; Nuñez, A.; Johnson, N. Temperate conditions restrict Japanese encephalitis virus infection to the mid-gut and prevents systemic dissemination in Culex pipiens mosquitoes. Sci. Rep. 2021, 11, 6133. [Google Scholar] [CrossRef]
- Garamazegi, L.Z. Climate change increases the risk of malaria in birds. Glob. Chang. Biol. 2011, 17, 1751–1759. [Google Scholar] [CrossRef]
- Loiseau, C.; Iezhova, T.; Valkiunas, G.; Chasar, A.; Hutchinson, A.; Buermann, W.; Smith, T.B.; Sehgal, R.N.M. Spatial variation of haemosporidian parasite infection in african rainforest bird species. J. Parasitol. 2010, 96, 21–29. [Google Scholar] [CrossRef]
- Sehgal, R.N.M.; Buermann, W.; Harrigan, R.J.; Bonneaud, C.; Loiseau, C.; Chasar, A.; Sepil, I.; Valkiunas, G.; Iezhova, T.; Saatchi, S.; et al. Spatially explicit predictions of blood parasites in a widely distributed African rainforest bird. Proc. R. Soc. B Biol. Sci. 2011, 278, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- LoGiudice, K.; Ostfeld, R.S.; Schmidt, K.A.; Keesing, F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. USA 2003, 100, 567–571. [Google Scholar] [CrossRef] [Green Version]
- Chua, K.B.; Goh, K.J.; Wong, K.T.; Kamarulzaman, A.; Tan, P.S.K.; Ksiazek, T.G.; Zaki, S.R.; Paul, G.; Lam, S.K.; Tan, C.T. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet 1999, 354, 1257–1259. [Google Scholar] [CrossRef]
- Tadei, W.P.; Thatcher, B.D.; Santos, J.M.M.; Scarpassa, V.M.; Rodrigues, I.B.; Rafael, M.S. Ecologic observations on anopheline vectors of malaria in the Brazilian amazon. Am. J. Trop. Med. Hyg. 1998, 59, 325–335. [Google Scholar] [CrossRef]
- Vittor, A.Y.; Gilman, R.H.; Tielsch, J.; Glass, G.; Shields, T.; Lozano, W.S.; Pinedo-Cancino, V.; Patz, J.A. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of Falciparum malaria in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2006, 74, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Ferraguti, M.; Hernández-Lara, C.; Sehgal, R.N.M.; Santiago-Alarcon, D. Anthropogenic effects on avian Haemosporidians and their vectors. In Avian Malaria and Related Parasites in the Tropics; Marzal, A., Santiago-Alarcon, D., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 451–485. [Google Scholar] [CrossRef]
- Hernández-Lara, C.; Carbó-Ramírez, P.; Santiago-Alarcon, D. Effects of land use change (rural-urban) on the diversity and epizootiological parameters of avian Haemosporida in a widespread neotropical bird. Acta Trop. 2020, 209, 105542. [Google Scholar] [CrossRef] [PubMed]
- Van Hoesel, W.; Marzal, A.; Magallanes, S.; Santiago-Alarcon, D.; Ibáñez-Bernal, S.; Renner, S. Management of ecosystems alters vector dynamics and haemosporidian infections. Sci. Rep. 2019, 9, 8779. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, M.; Martínez-de la Puente, J.; Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Effects of landscape anthropization on mosquito community composition and abundance. Sci. Rep. 2016, 6, 29002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Ponçon, N.; Balenghien, T.; Toty, C.; Ferré, J.B.; Thomas, C.; Dervieux, A.; L’Ambert, G.; Schaffner, F.; Bardin, O.; Fontenille, D. Effects of local anthropogenic changes on potential malaria vector Anopheles hyrcanus and West Nile virus vector Culex modestus, Camargue, France. Emerg. Infect. Dis. 2007, 13, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Brugman, V.A.; Hernández-Triana, L.M.; Medlock, J.M.; Fooks, A.R.; Carpenter, S.; Johnson, N. The role of Culex pipiens L. (Diptera: Culicidae) in virus transmission in Europe. Int. J. Environ. Res. Public Health 2018, 15, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Kilpatrick, A.M. “Bird biting” mosquitoes and human disease: A review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladeau, S.L.; Allan, B.F.; Leisnham, P.T.; Levy, M.Z. The ecological foundations of transmission potential and vector-borne disease in urban landscapes. Funct. Ecol. 2015, 29, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Bradley, C.; Altizer, S. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. Evol. 2007, 22, 95–102. [Google Scholar] [CrossRef]
- Gilioli, G.; Mariani, L. Sensitivity of Anopheles gambiae population dynamics to meteo-hydrological variability: A mechanistic approach. Malar. J. 2011, 10, 294. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Quevedo, C.; Davies, R.G.; Richardson, D.S. Predictors of malaria infection in a wild bird population: Landscape-level analyses reveal climatic and anthropogenic factors. J. Anim. Ecol. 2014, 83, 1091–1102. [Google Scholar] [CrossRef]
- Lopes, P.; Lourenco, P.; Sousa, C.; Novo, T.; Rodrigues, A.; Almeida, P.G.; Seixas, J. Modelling Patterns of Mosquito Density Based on Remote Sensing Images; Estoril Congress Center: Estoril, Portugal, 2005; pp. 251–258. [Google Scholar]
- Krama, T.; Krams, R.; Cīrule, D.; Moore, F.R.; Rantala, M.J.; Krams, I.A. Intensity of haemosporidian infection of parids positively correlates with proximity to water bodies, but negatively with host survival. Artic. J. Ornithol. 2015, 156, 1075–1084. [Google Scholar] [CrossRef]
- Chasar, A.; Loiseau, C.; Valkiūnas, G.; Iezhova, T.; Smith, T.B.; Sehgal, R.N.M. Prevalence and diversity patterns of avian blood parasites in degraded African rainforest habitats. Mol. Ecol. 2009, 18, 4121–4133. [Google Scholar] [CrossRef]
- Lüdtke, B.; Moser, I.; Santiago-Alarcon, D.; Fischer, M.; Kalko, E.K.V.; Martin Schaefer, H.; Suarez-Rubio, M.; Tschapka, M.; Renner, S.C. Associations of forest type, parasitism and body condition of two European passerines, Fringilla coelebs and Sylvia atricapilla. PLoS ONE 2013, 8, e81395. [Google Scholar] [CrossRef] [Green Version]
- Renner, S.C.; Bruntje, L.; Kaiser, S.; Kienle, J.; Schaefer, H.; Segelbacher, G.; Tschapk, M.; Santiago-Alarcon, D. Forests of opportunities and mischief: Disentangling the interactions between forests, parasites and immune responses. Int. J. Parasitol. 2016, 46, 571–579. [Google Scholar] [CrossRef]
- Bonneaud, C.; Sepil, I.; Mila, B.; Buermann, W.; Pollinger, J.; Sehgal, R.N.M.; Valkiūnas, G.; Iezhova, T.A.; Saatchi, S.; Smith, T.B. The prevalence of avian Plasmodium is higher in undisturbed tropical forests of Cameroon. J. Trop. Ecol. 2009, 25, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Reis, S.; Melo, M.; Covas, R.; Doutrelant, C.; Pereira, H.; de Lima, R.; Loiseau, C. Influence of land use and host species on parasite richness, prevalence and co-infection patterns. Int. J. Parasitol. 2021, 51, 83–94. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; Fernández-González, S.; de la Hera, I.; Pérez-Tris, J. Finding the appropriate variables to model the distribution of vector-borne parasites with different environmental preferences: Climate is not enough. Glob. Chang. Biol. 2013, 19, 3245–3253. [Google Scholar] [CrossRef]
- Muriel, J.; Marzal, A.; Magallanes, S.; García-Longoria, L.; Suarez-Rubio, M.; Bates, P.J.J.; Lin, H.H.; Soe, A.N.; Oo, K.S.; Aye, A.A.; et al. Prevalence and diversity of avian Haemosporidians may vary with anthropogenic disturbance in tropical habitats in Myanmar. Diversity 2021, 13, 111. [Google Scholar] [CrossRef]
- Van Hoesel, W.; Santiago-Alarcon, D.; Marzal, A.; Renner, S. Effects of forest structure on the interaction between avian hosts, dipteran vectors and haemosporidian parasites. BMC Ecol. 2020, 20, 47. [Google Scholar] [CrossRef]
- Richards, S.L.; Mores, C.N.; Lord, C.C.; Tabachnick, W.J. Impact of extrinsic incubation temperature and virus exposure on vector competence of Culex pipiens quinquefasciatus Say (Diptera: Culicidae) for West Nile virus. Vector-Borne Zoonotic Dis. 2007, 7, 629–636. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-López, R.; Martínez-de la Puente, J.; Gangoso, L.; Yan, J.; Soriguer, R.; Figuerola, J. Experimental reduction of host Plasmodium infection load affects mosquito survival. Sci. Rep. 2019, 9, 8782. [Google Scholar] [CrossRef]
- Ruiz, M.O.; Walker, E.D.; Foster, E.S.; Haramis, L.D.; Kitron, U.D. Association of West Nile virus illness and urban landscapes in Chicago and Detroit. Int. J. Health Geogr. 2007, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Paz, S.; Semenza, J. Environmental Drivers of West Nile Fever Epidemiology in Europe and Western Asia—A Review. Int. J. Environ. Res. Public Health 2013, 10, 3543–3562. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, S.E.J.; Wimberly, M.C.; Madden, M.; Masour, J.; Yabsley, M.J.; Stallknecht, D.E. Factors affecting the geographic distribution of West Nile virus in Georgia, USA: 2002–2004. Vector-Borne Zoonotic Dis. 2006, 6, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Komar, N.; Panella, N.A.; Langevin, S.A.; Brault, A.C.; Amador, M.; Edwards, E.; Owen, J.C. Avian hosts for West Nile virus in St. Tammany Parish, Louisiana 2002. Am. J. Trop. Med. Hyg. 2005, 73, 1031–1037. [Google Scholar] [CrossRef]
- Ringia, A.M.; Blitvich, B.J.; Koo, H.Y.; van de Wyngaerde, M.; Brawn, J.D.; Novak, R.J. Antibody prevalence of West Nile virus in birds, Illinois, 2002. Emerg. Infect. Dis. 2004, 10, 1120–1124. [Google Scholar] [CrossRef]
- Brown, H.E.; Childs, J.E.; Diuk-Wasser, M.A.; Fish, D. Ecological factors associated with West Nile virus transmission, northeastern United States. Emerg. Infect. Dis. 2008, 14, 1539–1545. [Google Scholar] [CrossRef]
- Vogels, C.B.F.; Fros, J.J.; Göertz, G.P.; Pijlman, G.P.; Koenraadt, C.J.M. Vector competence of northern European Culex pipiens biotypes and hybrids for West Nile virus is differentially affected by temperature. Parasit. Vectors 2016, 9, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wimberly, M.C.; Lamsal, A.; Giacomo, P.; Chuang, T.W. Regional variation of climatic influences on West Nile virus outbreaks in the United States. Am. J. Trop. Med. Hyg. 2014, 91, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Ciota, A.T.; Matacchiero, A.C.; Kilpatrick, A.M.; Kramer, L.D. The effect of temperature on life history traits of Culex mosquitoes. J. Med. Entomol. 2014, 51, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Golding, N.; Pigott, D.M.; Kraemer, M.U.G.; Messina, J.P.; Reiner, R.C.; Scott, T.W.; Smith, D.L.; Gething, P.W.; Hay, S.I. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit. Vectors 2014, 7, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpatrick, A.M.; Meola, M.A.; Moudy, R.M.; Kramer, L.D. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008, 4, e1000092. [Google Scholar] [CrossRef] [Green Version]
- Ruybal, J.E.; Kramer, L.D.; Kilpatrick, A.M. Geographic variation in the response of Culex pipiens life history traits to temperature. Parasit. Vectors 2016, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ewing, D.; Cobbold, C.; Purse, B.; Nunn, M.; White, S. Modelling the effect of temperature on the seasonal population dynamics of temperate mosquitoes. J. Biol. 2016, 400, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Reisen, W.K.; Fang, Y.; Martinez, V.M. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 2006, 43, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Rosà, R.; Marini, G.; Bolzoni, L.; Neteler, M.; Metz, M.; Delucchi, L.; Chadwick, E.A.; Balbo, L.; Mosca, A.; Giacobini, M.; et al. Early warning of West Nile virus mosquito vector: Climate and land use models successfully explain phenology and abundance of Culex pipiens mosquitoes in north-western Italy. Parasit. Vectors 2014, 7, 269. [Google Scholar] [CrossRef]
- Marcantonio, M.; Rizzoli, A.; Metz, M.; Rosa, R.; Marini, G.; Chadwick, E.; Neteler, M. Identifying the environmental conditions favouring West Nile virus outbreaks in Europe. PLoS ONE 2015, 10, e0121158. [Google Scholar] [CrossRef] [Green Version]
- Cotar, A.I.; Falcuta, E.; Prioteasa, L.F.; Dinu, S.; Ceianu, C.S.; Paz, S. Transmission dynamics of the West Nile virus in mosquito vector populations under the influence of weather factors in the Danube Delta, Romania. EcoHealth 2016, 13, 796–807. [Google Scholar] [CrossRef]
- Poh, K.C.; Chaves, L.F.; Reyna-Nava, M.; Roberts, C.M.; Fredregill, C.; Bueno, R.; Debboun, M.; Hamer, G.L. The influence of weather and weather variability on mosquito abundance and infection with West Nile virus in Harris County, Texas, USA. Sci. Total Environ. 2019, 675, 260–272. [Google Scholar] [CrossRef]
- Chuang, T.W.; Wimberly, M.C. Remote sensing of climatic anomalies and West Nile virus Incidence in the Northern Great Plains of the United States. PLoS ONE 2012, 7, e46882. [Google Scholar] [CrossRef]
- Shand, L.; Brown, W.M.; Chaves, L.F.; Goldberg, T.L.; Hamer, G.L.; Haramis, L.; Kitron, U.; Walker, E.D.; Ruiz, M.O. Predicting West Nile virus infection risk from the synergistic effects of rainfall and temperature. J. Med. Entomol. 2016, 53, 935–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Landscape effects on the presence, abundance and diversity of mosquitoes in mediterranean wetlands. PLoS ONE 2015, 10, 128112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo-Barriga, D.; Gomes, B.; Almeida, A.P.G.; Serrano-Aguilera, F.J.; Pérez-Martín, J.E.; Calero-Bernal, R.; Reina, D.; Frontera, E.; Pinto, J. The mosquito fauna of the western region of Spain with emphasis on ecological factors and the characterization of Culex pipiens forms. J. Vector Ecol. 2017, 42, 136–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz, S. Climate change impacts on West Nile virus transmission in a global context. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 1–11. [Google Scholar] [CrossRef]
- Roth, D.; Henry, B.; Mak, S.; Fraser, M.; Taylor, M.; Li, M.; Cooper, K.; Furnell, A.; Wong, Q.; Morshed, M. West Nile Virus range expansion into British Columbia. Emerg. Infect. Dis. 2010, 16, 1251–1258. [Google Scholar] [CrossRef]
- Loiseau, C.; Harrigan, R.J.; Cornel, A.J.; Guers, S.L.; Dodge, M.; Marzec, T.; Carlson, J.S.; Seppi, B.; Sehgal, R.N.M. First evidence and predictions of Plasmodium transmission in Alaskan bird populations. PLoS ONE 2012, 7, e44729. [Google Scholar] [CrossRef]
- Bataillard, D.; Christe, P.; Pigeault, R. Impact of field-realistic doses of glyphosate and nutritional stress on mosquito life history traits and susceptibility to malaria parasite infection. Ecol. Evol. 2020, 10, 5079–5088. [Google Scholar] [CrossRef]
- Hegde, S.; Rasgon, J.L.; Hughes, G.L. The microbiome modulates arbovirus transmission in mosquitoes. Curr. Opin. Virol. 2015, 15, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Martínez-de la Puente, J.; Gutiérrez-López, R.; Díez-Fernández, A.; Soriguer, R.C.; Moreno-Indias, I.; Figuerola, J. Effects of mosquito microbiota on the survival cost and development success of avian Plasmodium. Front. Microbiol. 2021, 11, 3406. [Google Scholar] [CrossRef]
- Manguin, S.; Boete, C. Global impact of mosquito biodiversity, human vector-borne diseases and environmental change. In The Importance of Biological Interactions in the Study of Biodiversity; IntechOpen: London, UK, 2011; pp. 27–50. [Google Scholar] [CrossRef] [Green Version]
- Martínez-de la Puente, J.; Ferraguti, M.; Ruiz, S.; Roiz, D.; Llorente, F.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Soriguer, R.; Figuerola, J. Mosquito community influences West Nile virus seroprevalence in wild birds: Implications for the risk of spillover into human populations. Sci. Rep. 2018, 8, 2599. [Google Scholar] [CrossRef] [Green Version]
- McKinney, M. Urbanization, biodiversity, and conservation. Bioscience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- Schrama, M.; Hunting, E.R.; Beechler, B.R.; Guarido, M.M.; Govender, D.; Nijland, W.; van‘t Zelfde, M.; Venter, M.; van Bodegom, P.M.; Gorsich, E.E. Human practices promote presence and abundance of disease-transmitting mosquito species. Sci. Rep. 2020, 10, 13546. [Google Scholar] [CrossRef] [PubMed]
- Byrne, K.; Nichols, R.A. Culex pipiens in London Underground tunnels: Differentiation between surface and subterranean populations. Heredity 1999, 82, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, B.H.; Ryan, P.A.; Russell, B.M.; Holt, J.S.; Lyons, S.A.; Foley, P.N. The importance of subterranean mosquito habitat to arbovirus vector control strategies in North Queensland, Australia. J. Med. Entomol. 2000, 37, 846–853. [Google Scholar] [CrossRef]
- Overgaard, H.J.; Ekbom, B.; Suwonkerd, W.; Takagi, M. Effect of landscape structure on anopheline mosquito density and diversity in northern Thailand: Implications for malaria transmission and control. Landsc. Ecol. 2003, 18, 605–619. [Google Scholar] [CrossRef]
- Gangoso, L.; Aragonés, D.; Martínez-de la Puente, J.; Lucientes, J.; Delacour-Estrella, S.; Estrada Peña, R.; Montalvo, T.; Bueno-Marí, R.; Bravo-Barriga, D.; Frontera, E.; et al. Determinants of the current and future distribution of the West Nile virus mosquito vector Culex pipiens in Spain. Environ. Res. 2020, 188, 109837. [Google Scholar] [CrossRef]
- Li, Y.; Kamara, F.; Zhou, G.; Puthiyakunnon, S.; Li, C.; Liu, Y.; Zhou, Y.; Yao, L.; Yan, G.; Chen, X.G. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl. Trop. Dis. 2014, 8, e3301. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, K.S.; Muturi, E.J.; Lampman, R.L.; Alto, R.W. The effects of resource type and ratio on competition with Aedes albopictus and Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2011, 48, 29–38. [Google Scholar] [CrossRef]
- Bevins, S.N. Timing of resource input and larval competition between invasive and native container-inhabiting mosquitoes (Diptera: Culicidae). J. Vector Ecol. 2007, 32, 252–267. [Google Scholar] [CrossRef]
- Marini, G.; Guzzetta, G.; Baldacchino, F.; Arnoldi, D.; Montarsi, F.; Capelli, G.; Rizzoli, A.; Merler, S.; Rosà, R. The effect of interspecific competition on the temporal dynamics of Aedes albopictus and Culex pipiens. Parasit. Vectors 2017, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Frankie, G.W.; Ehler, L.E. Ecology of insects in urban environments. Annu. Rev. Entomol. 1978, 23, 367–387. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Milheim, L.E.; Coffey, M.F.; Godsey, M.S.; King, R.J.; Guptill, S.C. Land cover variation and West Nile virus prevalence: Patterns, processes, and implications for disease control. Vector Borne Zoonotic Dis. 2007, 7, 173–180. [Google Scholar] [CrossRef]
- Takken, W.; Verhulst, N.O. Host preferences of blood-feeding mosquitoes. Annu. Rev. Entomol. 2013, 58, 433–453. [Google Scholar] [CrossRef] [Green Version]
- Sawabe, K.; Isawa, H.; Hoshino, K.; Sasaki, T.; Roychoudhury, S.; Higa, Y.; Kasai, S.; Tsuda, Y.; Nishiumi, I.; Hisai, N.; et al. Host-feeding habits of Culex pipiens and Aedes albopictus (Diptera: Culicidae) collected at the urban and suburban residential areas of Japan. J. Med. Entomol. 2010, 47, 442–450. [Google Scholar] [CrossRef] [Green Version]
- Valerio, L.; Marini, F.; Bongiorno, G.; Facchinelli, L.; Pombi, M.; Caputo, B.; Maroli, M.; Della Torre, A. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in urban and rural contexts within Rome province, Italy. Vector Borne Zoonotic Dis. 2010, 10, 291–294. [Google Scholar] [CrossRef]
- Faraji, A.; Egizi, A.; Fonseca, D.M.; Unlu, I.; Crepeau, T.; Healy, S.P.; Gaugler, R. Comparative host feeding patterns of the Asian tiger mosquito, Aedes albopictus, in urban and suburban northeastern USA and implications for disease transmission. PLoS Negl. Trop. Dis. 2014, 8, e3037. [Google Scholar] [CrossRef] [Green Version]
- Martínez-de la Puente, J.; Ferraguti, M.; Ruiz, S.; Roiz, D.; Soriguer, R.C.; Figuerola, J. Culex pipiens forms and urbanization: Effects on blood feeding sources and transmission of avian Plasmodium. Malar. J. 2016, 15, 589. [Google Scholar] [CrossRef] [Green Version]
- Martínez-de la Puente, J.; Muñoz, J.; Capelli, G.; Montarsi, F.; Soriguer, R.; Arnoldi, D.; Rizzoli, A.; Figuerola, J. Avian malaria parasites in the last supper: Identifying encounters between parasites and the invasive Asian mosquito tiger and native mosquito species in Italy. Malar. J. 2015, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Martínez-de la Puente, J.; Díez-Fernández, A.; Montalvo, T.; Bueno-Marí, R.; Pangrani, Q.; Soriguer, R.C.; Senar, J.C.; Figuerola, J. Do invasive mosquito and bird species alter avian malaria parasite transmission? Diversity 2020, 12, 111. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Tsuda, Y.; Yamada, A. Bloodmeal identification and detection of avian malaria parasite from mosquitoes (Diptera: Culicidae) inhabiting coastal areas of Tokyo bay, Japan. J. Med. Entomol. 2009, 46, 1230–1234. [Google Scholar] [CrossRef] [Green Version]
- Beerntsen, B.T.; James, A.A.; Christensen, B.M. Genetics of mosquito vector competence. Microbiol. Mol. Biol. Rev. 2000, 64, 115–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-López, R.; Martínez-de la Puente, J.; Gangoso, L.; Yan, J.; Soriguer, R.C.; Figuerola, J. Do mosquitoes transmit the avian malaria-like parasite Haemoproteus? An experimental test of vector competence using mosquito saliva. Parasit. Vectors 2016, 9, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zana, B.; Erdélyi, K.; Nagy, A.; Mezei, E.; Nagy, O.; Takács, M.; Bakonyi, T.; Forgách, P.; Korbacska-Kutasi, O.; Fehér, O.; et al. Multi-approach investigation regarding the West Nile virus situation in Hungary, 2018. Viruses 2020, 12, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-de la Puente, J.; Martínez, J.; Rivero-De Aguilar, J.; Herrero, J.; Merino, S. On the specificity of avian blood parasites: Revealing specific and generalist relationships between haemosporidians and biting midges. Mol. Ecol. 2011, 20, 3275–3287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Žiegyte, R.; Bernotiene, R.; Bukauskaite, D.; Palinauskas, V.; Iezhova, T.; Valkiunas, G. Complete sporogony of Plasmodium relictum (lineages pSGS1 and pGRW11) in mosquito Culex pipiens pipiens form molestus, with implications to avian malaria epidemiology. J. Parasitol. 2014, 100, 878–882. [Google Scholar] [CrossRef]
- Huppert, A.; Katriel, G. Mathematical modelling and prediction in infectious disease epidemiology. Clin. Microbiol. Infect. 2013, 19, 999–1005. [Google Scholar] [CrossRef] [Green Version]
- Olsson-Pons, S.; Clark, N.J.; Ishtiaq, F.; Clegg, S.M. Differences in host species relationships and biogeographic influences produce contrasting patterns of prevalence, community composition and genetic structure in two genera of avian malaria parasites in southern Melanesia. J. Anim. Ecol. 2015, 84, 985–998. [Google Scholar] [CrossRef]
- Garcia-Longoria, L.; Marzal, A.; de Lope, F.; Garamszegi, L. Host-parasite interaction explains variation in the prevalence of avian haemosporidians at the community level. PLoS ONE 2019, 14, e0205624. [Google Scholar] [CrossRef]
- Ferraguti, M. Biodiversity and Vector-Borne Diseases: Effects of Landscape, Mosquito and Vertebrate Communities on the Transmission of West Nile Virus and Avian Malaria Parasites; Universidad Pablo de Olavide: Seville, Spain, 2017. [Google Scholar]
- Johnson, P.T.J.; Wood, C.L.; Joseph, M.B.; Preston, D.L.; Haas, S.E.; Springer, Y.P. Habitat heterogeneity drives the host-diversity-begets-parasite-diversity relationship: Evidence from experimental and field studies. Ecol. Lett. 2016, 19, 752–761. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraguti, M.; Martínez-de la Puente, J.; Figuerola, J. Ecological Effects on the Dynamics of West Nile Virus and Avian Plasmodium: The Importance of Mosquito Communities and Landscape. Viruses 2021, 13, 1208. https://doi.org/10.3390/v13071208
Ferraguti M, Martínez-de la Puente J, Figuerola J. Ecological Effects on the Dynamics of West Nile Virus and Avian Plasmodium: The Importance of Mosquito Communities and Landscape. Viruses. 2021; 13(7):1208. https://doi.org/10.3390/v13071208
Chicago/Turabian StyleFerraguti, Martina, Josué Martínez-de la Puente, and Jordi Figuerola. 2021. "Ecological Effects on the Dynamics of West Nile Virus and Avian Plasmodium: The Importance of Mosquito Communities and Landscape" Viruses 13, no. 7: 1208. https://doi.org/10.3390/v13071208
APA StyleFerraguti, M., Martínez-de la Puente, J., & Figuerola, J. (2021). Ecological Effects on the Dynamics of West Nile Virus and Avian Plasmodium: The Importance of Mosquito Communities and Landscape. Viruses, 13(7), 1208. https://doi.org/10.3390/v13071208