West Nile Virus and Tick-Borne Encephalitis Virus Are Endemic in Equids in Eastern Austria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Questionnaire and Data Collection
2.3. Flavivirus Antibody Detection in Serum Samples
2.4. WNV RNA Detection in Serum Samples
2.5. Data Analysis and Statistical Methods
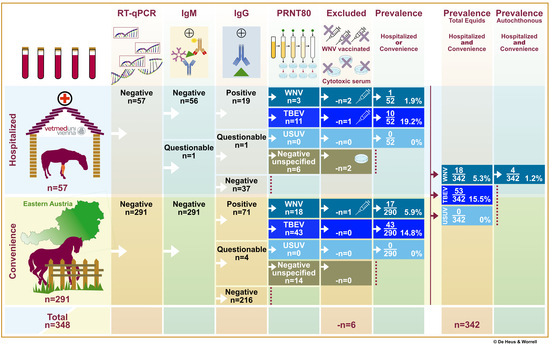
3. Results
3.1. Study Design and Population
3.2. Laboratory Results
3.2.1. Flavivirus Antibody Detection in Serum Samples
3.2.2. Austrian Autochthonous WNV Infections
3.2.3. Detection of WNV Nucleic Acid in Equine Serum
3.3. Geographic Distribution
3.4. Risk Factor Analysis
4. Discussion
4.1. West Nile Virus
4.2. Tick-Borne Encephalitis Virus
4.3. Usutu Virus
4.4. Donkey
4.5. Risk Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weissenböck, H.; Kolodziejek, J.; Url, A.; Lussy, H.; Rebel-Bauder, B.; Nowotny, N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg. Infect. Dis. 2002, 8, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Weissenböck, H.; Bakonyi, T.; Rossi, G.; Mani, P.; Nowotny, N. Usutu virus, Italy, 1996. Emerg. Infect. Dis. 2013, 19, 274–277. [Google Scholar] [CrossRef]
- Bakonyi, T.; Ivanics, É.; Erdélyi, K.; Ursu, K.; Ferenczi, E.; Weissenböck, H.; Nowotny, N. Lineage 1 and 2 strains of encephalitic West Nile virus, Central Europe. Emerg. Infect. Dis. 2006, 12, 618–623. [Google Scholar] [CrossRef]
- Rizzoli, A.; Jimenez-Clavero, M.A.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The challenge of West Nile virus in Europe: Knowledge gaps and research priorities. Eurosurveillance 2015, 20, 21135. [Google Scholar] [CrossRef] [Green Version]
- Barzon, L.; Papa, A.; Lavezzo, E.; Franchin, E.; Pacenti, M.; Sinigaglia, A.; Masi, G.; Trevisan, M.; Squarzon, L.; Toppo, S.; et al. Phylogenetic characterization of Central/Southern European lineage 2 West Nile virus: Analysis of human outbreaks in Italy and Greece, 2013–2014. Clin. Microbiol. Infect. 2015, 21, 1122.e1–1122.e10. [Google Scholar] [CrossRef] [Green Version]
- Gossner, C.M.; Marrama, L.; Carson, M.; Allerberger, F.; Calistri, P.; Dilaveris, D.; Lecollinet, S.; Morgan, D.; Nowotny, N.; Paty, M.C.; et al. West Nile virus surveillance in Europe: Moving towards an integrated animal-human-vector approach. Eurosurveillance 2017, 22, 30526. [Google Scholar] [CrossRef] [PubMed]
- Domanović, D.; Gossner, C.M.; Lieshout-Krikke, R.; Mayr, W.; Baroti-Toth, K.; Dobrota, A.M.; Escoval, M.A.; Henseler, O.; Jungbauer, C.; Liumbruno, G.; et al. West Nile and Usutu virus infections and challenges to blood safety in the European Union. Emerg. Infect. Dis. 2019, 25, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejek, J.; Seidel, B.; Jungbauer, C.; Dimmel, K.; Kolodziejek, M.; Rudolf, I.; Hubálek, Z.; Allerberger, F.; Nowotny, N. West Nile virus positive blood donation and subsequent entomological investigation, Austria, 2014. PLoS ONE 2015, 10, e0126381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aberle, S.W.; Stiasny, K.; Aberle, J.H.; Kolodziejek, J.; Nowotny, N.; Jungbauer, C.; Weidner, L.; Zoufaly, A.; Hourfar, M.K. Increase in human West Nile and Usutu virus infections, Austria, 2018. Eurosurveillance 2018, 23, 1800545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinz, F.X.; Stiasny, K.; Holzmann, H.; Kundi, M.; Sixl, W.; Wenk, M.; Kainz, W.; Essl, A.; Kunz, C. Emergence of tick-borne encephalitis in new endemic areas in Austria: 42 years of surveillance. Eurosurveillance 2015, 20, 21077. [Google Scholar] [CrossRef] [Green Version]
- Camp, J.V.; Nowotny, N. The knowns and unknowns of West Nile virus in Europe: What did we learn from the 2018 outbreak? Expert Rev. Anti-Infect. Ther. 2020, 18, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, P.; Kolodziejek, J.; Bakonyi, T.; Brunthaler, R.; Erdélyi, K.; Weissenböck, H.; Nowotny, N. Different dynamics of Usutu virus infections in Austria and Hungary, 2017–2018. Transbound. Emerg. Dis. 2020, 67, 298–307. [Google Scholar] [CrossRef]
- Bakran-Lebl, K.; Camp, J.; Kolodziejek, J.; Weidinger, P.; Hufnagl, P.; Cabal Rosel, A.; Zwickelstorfer, A.; Allerberger, F.; Nowotny, N. Diversity of West Nile and Usutu virus strains in mosquitoes at an international airport in Austria. Transbound. Emerg. Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, Z.; Rudolf, I.; Nowotny, N. Arboviruses pathogenic for domestic and wild animals. Adv. Virus Res. 2014, 89, 201–275. [Google Scholar] [PubMed]
- Rudolf, I.; Bakonyi, T.; Šebesta, O.; Mendel, J.; Peško, J.; Betášová, L.; Blažejová, H.; Venclíková, K.; Straková, P.; Nowotny, N.; et al. Co-circulation of Usutu virus and West Nile virus in a reed bed ecosystem. Parasites Vectors 2015, 8, 520. [Google Scholar] [CrossRef] [Green Version]
- Kolodziejek, J.; Jungbauer, C.; Aberle, S.W.; Allerberger, F.; Bagó, Z.; Camp, J.V.; Dimmel, K.; De Heus, P.; Kolodziejek, M.; Schiefer, P.; et al. Integrated analysis of human-animal-vector surveillance: West Nile virus infections in Austria, 2015–2016. Emerg. Microbes Infect. 2018, 7, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camp, J.V.; Kolodziejek, J.; Nowotny, N. Targeted surveillance reveals native and invasive mosquito species infected with Usutu virus. Parasites Vectors 2019, 12, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakonyi, T.; Ferenczi, E.; Erdélyi, K.; Kutasi, O.; Csörgo, T.; Seidel, B.; Weissenböck, H.; Brugger, K.; Bán, E.; Nowotny, N. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet. Microbiol. 2013, 165, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Wodak, E.; Richter, S.; Bagó, Z.; Revilla-Fernández, S.; Weissenböck, H.; Nowotny, N.; Winter, P. Detection and molecular analysis of West Nile virus infections in birds of prey in the eastern part of Austria in 2008 and 2009. Vet. Microbiol. 2011, 149, 358–366. [Google Scholar] [CrossRef] [Green Version]
- De Heus, P.; Kolodziejek, J.; Camp, J.V.; Dimmel, K.; Bagó, Z.; Van den Hoven, R.; Cavalleri, J.-M.V.; Nowotny, N. Emergence of West Nile virus lineage 2 in Europe: Characteristics of the first seven cases of West Nile neuroinvasive disease in horses in Austria. Transbound. Emerg. Dis. 2019, 67, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Weissenböck, H.; Kolodziejek, J.; Fragner, K.; Kuhn, R.; Pfeffer, M.; Nowotny, N. Usutu virus activity in Austria, 2001–2002. Microbes Infect. 2003, 5, 1132–1136. [Google Scholar] [CrossRef]
- Pecorari, M.; Longo, G.; Gennari, W.; Grottola, A.; Sabbatini, A.; Tagliazucchi, S.; Savini, G.; Monaco, F.; Simone, M.; Lelli, R.; et al. First human case of Usutu virus neuroinvasive infection, Italy, August–September 2009. Eurosurveillance 2009, 14, 19446. [Google Scholar] [CrossRef]
- Cavrini, F.; Gaibani, P.; Longo, G.; Pierro, A.M.; Rossini, G.; Bonilauri, P.; Gerunda, G.; Di Benedetto, F.; Pasetto, A.; Girardis, M.; et al. Usutu virus infection in a patient who underwent orthotropic liver transplantation, Italy, August–September 2009. Eurosurveillance 2009, 14, 19448. [Google Scholar] [CrossRef]
- Nagy, A.; Mezei, E.; Nagy, O.; Bakonyi, T.; Csonka, N.; Kaposi, M.; Koroknai, A.; Szomor, K.; Rigó, Z.; Molnár, Z.; et al. Extraordinary increase in West Nile virus cases and first confirmed human Usutu virus infection in Hungary, 2018. Eurosurveillance 2019, 24, 1900038. [Google Scholar] [CrossRef] [Green Version]
- Bakonyi, T.; Kolodziejek, J.; Dimmel, K.; Nowotny, N.; Jungbauer, C.; Aberle, S.W.; Stiasny, K.; Allerberger, F. Usutu virus infections among blood donors, Austria, July and August 2017—Raising awareness for diagnostic challenges. Eurosurveillance 2017, 22, 17-00644. [Google Scholar] [CrossRef]
- Barbic, L.; Vilibic-Cavlek, T.; Listes, E.; Stevanovic, V.; Gjenero-Margan, I.; Ljubin-Sternak, S.; Pem-Novosel, I.; Listes, I.; Mlinaric-Galinovic, G.; Di Gennaro, A.; et al. Demonstration of Usutu virus antibodies in horses, Croatia. Vector-Borne Zoonotic Dis. 2013, 13, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Bażanów, B.; Jansen van Vuren, P.; Szymański, P.; Stygar, D.; Frącka, A.; Twardoń, J.; Kozdrowski, R.; Pawęska, J.T. A survey on West Nile and Usutu viruses in horses and birds in Poland. Viruses 2018, 10, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savini, G.; Monaco, F.; Terregino, C.; Di Gennaro, A.; Bano, L.; Pinoni, C.; De Nardi, R.; Bonilauri, P.; Pecorari, M.; Di Gialleonardo, L.; et al. Usutu virus in Italy: An emergence or a silent infection? Vet. Microbiol. 2011, 151, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Michelitsch, A.; Wernike, K.; Klaus, C.; Dobler, G.; Beer, M. Exploring the reservoir hosts of tick-borne encephalitis virus. Viruses 2019, 11, 669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunz, C. TBE vaccination and the Austrian experience. Vaccine 2003, 21, S50–S55. [Google Scholar] [CrossRef]
- Rushton, J.O.; Lecollinet, S.; Hubálek, Z.; Svobodová, P.; Lussy, H.; Nowotny, N. Tick-borne encephalitis virus in horses, Austria, 2011. Emerg. Infect. Dis. 2013, 19, 635–637. [Google Scholar] [CrossRef]
- Van Maanen, K.; Reusken, C.; Reimerink, J.; Van der Heijden, H. Surveillance of West Nile virus incursions in the Netherlands: Validation of antibody detection ELISAs in horses. In Proceedings of the Oral Presentation at the 2010 Epizone Meeting in Saint Malo, Saint-Malo, France, 7–10 June 2010. [Google Scholar]
- Hubálek, Z.; Kříž, B.; Halouzka, J. Serologic survey of humans for flavivirus West Nile in Southern Moravia (Czech Republic). Cent. Eur. J. Public Health 2011, 19, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Badenhorst, M.; de Heus, P.; Auer, A.; Rümenapf, T.; Tegtmeyer, B.; Kolodziejek, J.; Nowotny, N.; Steinmann, E.; Cavalleri, J.M.V. No evidence of mosquito involvement in the transmission of Equine Hepacivirus (Flaviviridae) in an epidemiological survey of Austrian horses. Viruses 2019, 11, 1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolodziejek, J.; Marinov, M.; Kiss, B.J.; Alexe, V.; Nowotny, N. The complete sequence of a West Nile virus lineage 2 strain detected in a Hyalomma marginatum marginatum tick collected from a song thrush (Turdus philomelos) in Eastern Romania in 2013 revealed closest genetic relationship to strain Volgograd 2007. PLoS ONE 2014, 9, e109905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paz, S.; Semenza, J.C. Environmental drivers of West Nile fever epidemiology in Europe and Western Asia—A review. Int. J. Environ. Res. Public Health 2013, 10, 3543–3562. [Google Scholar] [CrossRef] [Green Version]
- Aharonson-Raz, K.; Lichter-Peled, A.; Tal, S.; Gelman, B.; Cohen, D.; Klement, E.; Steinman, A. Spatial and temporal distribution of West Nile virus in horses in Israel (1997–2013)-From endemic to epidemics. PLoS ONE 2014, 9, e113149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilpatrick, A.M.; Meola, M.A.; Moudy, R.M.; Kramer, L.D. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008, 4, e1000092. [Google Scholar] [CrossRef] [Green Version]
- Zentralanstalt Für Metereologie und Geodynamik. Available online: https://www.zamg.ac.at/cms/de/dokumente/topmenu/jahresberichte/jahresbericht_2017 (accessed on 6 June 2021).
- Vogelgesang, J.R.; Walter, M.; Kahl, O.; Rubel, F.; Brugger, K. Long-term monitoring of the seasonal density of questing ixodid ticks in Vienna (Austria): Setup and first results. Exp. Appl. Acarol. 2020, 81, 409–420. [Google Scholar] [CrossRef]
- Beck, C.; Jimenez-Clavero, M.A.; Leblond, A.; Durand, B.; Nowotny, N.; Leparc-Goffart, I.; Zientara, S.; Jourdain, E.; Lecollinet, S. Flaviviruses in Europe: Complex circulation patterns and their consequences for the diagnosis and control of West Nile disease. Int. J. Environ. Res. Public Health 2013, 10, 6049–6083. [Google Scholar] [CrossRef] [Green Version]
- Beck, C.; Desprès, P.; Paulous, S.; Vanhomwegen, J.; Lowenski, S.; Nowotny, N.; Durand, B.; Garnier, A.; Blaise-Boisseau, S.; Guitton, E.; et al. A High-performance multiplex immunoassay for serodiagnosis of flavivirus-associated neurological diseases in horses. BioMed Res. Int. 2015, 2015, 678084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleton, N.B.; van Maanen, K.; Bergervoet, S.A.; Bon, N.; Beck, C.; Godeke, G.J.; Lecollinet, S.; Bowen, R.; Lelli, D.; Nowotny, N.; et al. A serological protein microarray for detection of multiple cross-reactive flavivirus infections in horses for veterinary and public health surveillance. Transbound. Emerg. Dis. 2017, 64, 1801–1812. [Google Scholar] [CrossRef] [PubMed]
- Rockstroh, A.; Moges, B.; Berneck, B.S.; Sattler, T.; Revilla-Fernández, S.; Schmoll, F.; Pacenti, M.; Sinigaglia, A.; Barzon, L.; Schmidt-Chanasit, J.; et al. Specific detection and differentiation of tick-borne encephalitis and West Nile virus induced IgG antibodies in humans and horses. Transbound. Emerg. Dis. 2019, 66, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Girl, P.; Bestehorn-Willmann, M.; Zange, S.; Borde, J.P.; Dobler, G.; Von Buttlar, H. Tick-borne encephalitis virus nonstructural protein 1 IgG enzyme-linked immunosorbent assay for differentiating infection versus vaccination antibody responses. J. Clin. Microbiol. 2020, 58, e01783-19. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health (OIE); Monaco, F.; Sturgill, T. Chapter 3.1.24. West Nile Fever. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2018; Office International des Epizooties: Paris, France, 2018; Volume 1–3, pp. 697–710. [Google Scholar]
- World Organisation for Animal Health (OIE); Yang, D.-K. Chapter 3.1.10 Japanese encephalitis. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2018; Office International des Epizooties: Paris, France, 2018; Volume 1–3, pp. 477–490. [Google Scholar]
- Weissenböck, H.; Hubálek, Z.; Halouzka, J.; Pichlmair, A.; Maderner, A.; Fragner, K.; Kolodziejek, J.; Loupal, G.; Koelbl, K.; Nowotny, N. Screening for West Nile virus infections of susceptible animal species in Austria. Epidemiol. Infect. 2003, 131, 1023–1027. [Google Scholar] [CrossRef]
- Ostlund, E.N.; Crom, R.L.; Pedersen, D.D.; Johnson, D.J.; Williams, W.O.; Schmitt, B.J. Equine West Nile encephalitis, United States. Emerg. Infect. Dis. 2001, 7, 665–669. [Google Scholar] [CrossRef]
- Kutasi, O.; Bakonyi, T.; Lecollinet, S.; Biksi, I.; Ferenczi, E.; Bahuon, C.; Sardi, S.; Zientara, S.; Szenci, O. Equine encephalomyelitis outbreak caused by a genetic lineage 2 West Nile virus in Hungary. J. Vet. Intern. Med. 2011, 25, 586–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Centre for Disease Prevention and Control (ECDC). Human and Equine West Nile Fever Cases in Europe and the Mediterranean, 2017 Transmission Season. Available online: https://www.ecdc.europa.eu/en/publications-data/human-and-equine-west-nile-fever-cases-europe-and-mediterranean-2017-transmission (accessed on 6 June 2021).
- Hubálek, Z.; Ludvíková, E.; Jahn, P.; Treml, F.; Rudolf, I.; Svobodová, P.; Šikutová, S.; Betášová, L.; Bíreš, J.; Mojžíš, M.; et al. West Nile Virus equine serosurvey in the Czech and Slovak republics. Vector Borne Zoonotic Dis. 2013, 13, 733–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilibic-Cavlek, T.; Savic, V.; Petrovic, T.; Toplak, I.; Barbic, L.; Petric, D.; Tabain, I.; Hrnjakovic-Cvjetkovic, I.; Bogdanic, M.; Klobucar, A.; et al. Emerging trends in the epidemiology of West Nile and Usutu virus infections in Southern Europe. Front. Vet. Sci. 2019, 6, 437. [Google Scholar] [CrossRef] [Green Version]
- Medić, S.; van den Hoven, R.; Petrović, T.; Lupulović, D.; Nowotny, N. Serological evidence of West Nile virus infection in the horse population of northern Serbia. J. Infect. Dev. Ctries. 2014, 8, 914–918. [Google Scholar] [CrossRef] [Green Version]
- Papa, A.; Bakonyi, T.; Xanthopoulou, K.; Vázquez, A.; Tenorio, A.; Nowotny, N. Genetic characterization of West Nile virus lineage 2, Greece, 2010. Emerg. Infect. Dis. 2011, 17, 920–922. [Google Scholar] [CrossRef]
- Ziegler, U.; Lühken, R.; Keller, M.; Cadar, D.; van der Grinten, E.; Michel, F.; Albrecht, K.; Eiden, M.; Rinder, M.; Lachmann, L.; et al. West Nile virus epizootic in Germany, 2018. Antivir. Res. 2019, 162, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Young, J.J.; Coulombier, D.; Domanović, D.; European Union West Nile Fever Working Group; Zeller, H.; Gossner, C.M. One Health approach for West Nile virus surveillance in the European Union: Relevance of equine data for blood safety. Eurosurveillance 2019, 24, 1800349. [Google Scholar] [CrossRef] [PubMed]
- Luckschander, N.; Kölbl, S.; Enzesberger, O.; Zipko, H.T.; Thalhammer, J.G. Frühsommermeningoenzephalitis-(FSME-) Infektion in einer österreichischen Pferdepopulation. Tierärztl. Prax. 1999, 27, 235–238. [Google Scholar]
- Klaus, C.; Hörügel, U.; Hoffmann, B.; Beer, M. Tick-borne encephalitis virus (TBEV) infection in horses: Clinical and laboratory findings and epidemiological investigations. Vet. Microbiol. 2013, 163, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; König, M.; Thiel, H.J. Die Tick-borne enzephalitis (TBE) unter besonderer Berücksichtigung der Infektion beim Pferd. Dtsch. Tierarztl. Wochenschr. 2006, 113, 147–151. [Google Scholar] [PubMed]
- Csank, T.; Drzewnioková, P.; Korytár, L.; Major, P.; Gyuranecz, M.; Pistl, J.; Bakonyi, T. A serosurvey of flavivirus infection in horses and birds in Slovakia. Vector-Borne Zoonotic Dis. 2018, 18, 206–213. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Surveillance Report Tick-Borne Encephaltitis Annual Epidemiological Report for 2017. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-tick-borne-encephalitis_0.pdf (accessed on 31 August 2021).
- Vanhomwegen, J.; Beck, C.; Desprès, P.; Figuerola, A.; García, R.; Lecollinet, S.; López-Roig, M.; Manuguerra, J.C.; Serra-Cobo, J. Circulation of zoonotic arboviruses in equine populations of Mallorca Island (Spain). Vector-Borne Zoonotic Dis. 2017, 17, 340–346. [Google Scholar] [CrossRef]
- Lupulovic, D.; Martín-Acebes, M.A.; Lazic, S.; Alonso-Padilla, J.; Blázquez, A.-B.; Escribano-Romero, E.; Petrovic, T.; Saiz, J.-C. First serological evidence of West Nile virus activity in horses in Serbia. Vector-Borne Zoonotic Dis. 2011, 11, 1303–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.S.G.; Matos, A.C.D.; da Cunha, M.A.C.R.; Rehfeld, I.S.; Galinari, G.C.F.; Marcelino, S.A.C.; Saraiva, L.H.G.; Martins, N.R.D.S.; Maranhão, R.D.P.A.; Lobato, Z.I.P.; et al. West Nile virus associated with equid encephalitis in Brazil, 2018. Transbound. Emerg. Dis. 2019, 66, 445–453. [Google Scholar] [CrossRef] [Green Version]
- García-Bocanegra, I.; Arenas-Montes, A.; Jaén-Téllez, J.A.; Napp, S.; Fernández-Morente, M.; Arenas, A. Use of sentinel serosurveillance of mules and donkeys in the monitoring of West Nile virus infection. Vet. J. 2012, 194, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Širmarová, J.; Tichá, L.; Golovchenko, M.; Salát, J.; Grubhoffer, L.; Rudenko, N.; Nowotny, N.; Růžek, D. Seroprevalence of Borrelia burgdorferi sensu lato and tick-borne encephaltitis virus in zoo animal species in the Czech Republic. Ticks Tick-Borne Dis. 2014, 5, 523–527. [Google Scholar] [CrossRef]
- Zohaib, A.; Saqib, M.; Beck, C.; Hussain, M.H.; Lowenski, S.; Lecollinet, S.; Sial, A.; Asi, M.N.; Mansoor, M.K.; Saqalein, M.; et al. High prevalence of West Nile virus in equines from the two provinces of Pakistan. Epidemiol. Infect. 2015, 143, 1931–1935. [Google Scholar] [CrossRef] [PubMed]
- Abutarbush, S.M.; Al-Majali, A.M. West Nile virus infection in horses in Jordan: Clinical cases, seroprevalence and risk factors. Transbound. Emerg. Dis. 2014, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ahmadnejad, F.; Otarod, V.; Fallah, M.H.; Lowenski, S.; Sedighi-Moghaddam, R.; Zavareh, A.; Durand, B.; Lecollinet, S.; Sabatier, P. Spread of West Nile virus in Iran: A cross-sectional serosurvey in equines, 2008-2009. Epidemiol. Infect. 2011, 139, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, M.M.; Stringer, A.P.; Roess, A.A. Seroprevalence, risk factors and spatial distribution of West Nile virus in Jordan. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Salazar, P.; Traub-Dargatz, J.L.; Morley, P.S.; Wilmot, D.D.; Steffen, D.J.; Cunningham, W.E.; Salman, M.D. Outcome of equids with clinical signs of West Nile virus infection and factors associated with death. J. Am. Vet. Med. Assoc. 2004, 225, 267–274. [Google Scholar] [CrossRef] [PubMed]
- García-Bocanegra, I.; Arenas-Montes, A.; Napp, S.; Jaén-Téllez, J.A.; Fernández-Morente, M.; Fernández-Molera, V.; Arenas, A. Seroprevalence and risk factors associated to West Nile virus in horses from Andalusia, Southern Spain. Vet. Microbiol. 2012, 160, 341–346. [Google Scholar] [CrossRef]
- Epp, T.; Waldner, C.; West, K.; Townsend, H. Factors associated with West Nile virus disease fatalities in horses. Can. Vet. J. 2007, 48, 1137–1145. [Google Scholar] [PubMed]
- Epp, T.; Waldner, C.; Townsend, H. A case-control study of factors associated with the development of clinical disease due to West Nile virus, Saskatchwan 2003. Equine Vet. J. 2007, 39, 498–503. [Google Scholar] [CrossRef]




| Population | Group Hosp Number (n) | Group Hosp Percentage (%) | Group Conv Number (n) | Group Conv Percentage (%) | |
|---|---|---|---|---|---|
| Breed | Arabian and cross | 1 | 1.8 | 13 | 4.5 |
| Donkey | 1 | 1.8 | 13 | 4.5 | |
| Haflinger and cross | 9 | 15.8 | 12 | 4.1 | |
| Icelandic horse | 4 | 7.0 | 5 | 1.7 | |
| Lipizzan | 0 | 0 | 12 | 4.1 | |
| Noriker | 3 | 5.3 | 7 | 2.4 | |
| Pony | 1 | 1.8 | 28 | 9.6 | |
| Quarter horse | 5 | 8.8 | 18 | 6.2 | |
| (Mini) Shetland pony | 1 | 1.8 | 14 | 4.8 | |
| Standardbred | 3 | 5.3 | 15 | 5.2 | |
| Thoroughbred and cross | 0 | 0 | 4 | 1.4 | |
| Warmblood | 22 | 38.6 | 130 | 44.7 | |
| Welsh pony and cross | 0 | 0 | 4 | 1.4 | |
| Other | 7 | 12.3 | 11 | 3.8 | |
| Unknown | 0 | 0 | 5 | 1.7 | |
| Gender | Mare | 26 | 45.6 | 117 | 40.2 |
| Gelding | 30 | 52.6 | 149 | 51.2 | |
| Stallion | 1 | 1.8 | 24 | 8.2 | |
| Not recorded | 0 | 0 | 1 | 0.3 | |
| Reason for hospital admission | Orthopedic | 21 | 36.8 | ||
| Gastrointestinal | 11 | 19.3 | |||
| Dental | 6 | 10.5 | |||
| Dermatologic | 4 | 7.0 | |||
| Ophthalmologic | 4 | 7.0 | |||
| Respiratory | 4 | 7.0 | |||
| Fever 1 | 2 | 3.5 | |||
| Urinary | 2 | 3.5 | |||
| Neurologic | 2 | 3.5 | |||
| Companion animal | 1 | 1.8 |
| Variable | Relative Risk Ratio Probable Agent | |
|---|---|---|
| WNV | TBEV | |
| Import (Yes vs. No) | 2.55 | 1.56 |
| Insect protection 1 (Yes vs. No) | 0.56 | 1.56 |
| Stable type (Outdoor vs. Box) | 1.20 | 0.92 |
| Coat color (Light vs. Dark) | 0.56 | 1.47 |
| Coat color (Twotone vs. Dark) 2 | 0.66 | 1.90 |
| Illness ≤ 12 months | 1.02 | 1.59 |
| Stable federal state 3 (LA-east vs. Vienna) (LA-west vs. Vienna) | 1.31 0.70 | 0.37 2.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Heus, P.; Kolodziejek, J.; Hubálek, Z.; Dimmel, K.; Racher, V.; Nowotny, N.; Cavalleri, J.-M.V. West Nile Virus and Tick-Borne Encephalitis Virus Are Endemic in Equids in Eastern Austria. Viruses 2021, 13, 1873. https://doi.org/10.3390/v13091873
de Heus P, Kolodziejek J, Hubálek Z, Dimmel K, Racher V, Nowotny N, Cavalleri J-MV. West Nile Virus and Tick-Borne Encephalitis Virus Are Endemic in Equids in Eastern Austria. Viruses. 2021; 13(9):1873. https://doi.org/10.3390/v13091873
Chicago/Turabian Stylede Heus, Phebe, Jolanta Kolodziejek, Zdenĕk Hubálek, Katharina Dimmel, Victoria Racher, Norbert Nowotny, and Jessika-M. V. Cavalleri. 2021. "West Nile Virus and Tick-Borne Encephalitis Virus Are Endemic in Equids in Eastern Austria" Viruses 13, no. 9: 1873. https://doi.org/10.3390/v13091873
APA Stylede Heus, P., Kolodziejek, J., Hubálek, Z., Dimmel, K., Racher, V., Nowotny, N., & Cavalleri, J. -M. V. (2021). West Nile Virus and Tick-Borne Encephalitis Virus Are Endemic in Equids in Eastern Austria. Viruses, 13(9), 1873. https://doi.org/10.3390/v13091873