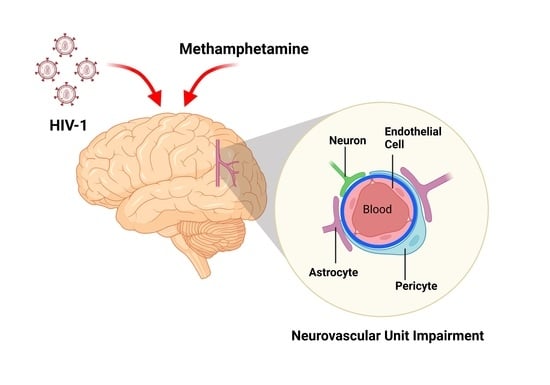
Synergistic Impairment of the Neurovascular Unit by HIV-1 Infection and Methamphetamine Use: Implications for HIV-1-Associated Neurocognitive Disorders
Abstract
:1. Introduction
2. Synergistic Impairment of the NVU Induced by Comorbid HIV-1 Infection and Methamphetamine Use
2.1. Disruption of Tight Junctions and Transporters of Brain Endothelial Cells
2.2. Oxidative Stress and Inflammation
2.3. Alterations in Astrocytes and Pericytes
2.4. Facilitation of Immune Cell Transmigration across the BBB
3. Potentiation of Neuroinflammation by the Combination of HIV-1 Infection and Methamphetamine Use
3.1. Mechanisms of Neuronal Dysfunction
3.2. Contribution of Microglia and Astrocytes to Neuroinflammation
4. Combined Effects of HIV-1 Infection and Methamphetamine Use on Neurocognitive Functioning
5. Developing New Therapeutic Approaches for Comorbid HIV-1 Infection and METH Use Disorder
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, A.; Neelamegam, M.; Rajasuriar, R. Ageing with HIV: Health implications and evolving care needs. J. Int. AIDS Soc. 2020, 23, e25621. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Lu, Q.; Farrell, M.; Lappin, J.M.; Shi, J.; Lu, L.; Bao, Y. Global prevalence and burden of HIV-associated neurocognitive disorder: A meta-analysis. Neurology 2020, 95, e2610–e2621. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, L.; Cho, H.J.; Toborek, M. Blood-brain barrier pericytes as a target for HIV-1 infection. Brain 2019, 142, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Lutgen, V.; Narasipura, S.D.; Barbian, H.J.; Richards, M.; Wallace, J.; Razmpour, R.; Buzhdygan, T.; Ramirez, S.H.; Prevedel, L.; Eugenin, E.A.; et al. HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLoS Pathog. 2020, 16, e1008381. [Google Scholar] [CrossRef]
- Wallet, C.; De Rovere, M.; Van Assche, J.; Daouad, F.; De Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Van Lint, C.; Rohr, O.; et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front. Cell. Infect. Microbiol. 2019, 9, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayyad, Z.; Spudich, S. Neuropathogenesis of HIV: From Initial Neuroinvasion to HIV-Associated Neurocognitive Disorder (HAND). Curr. HIV/AIDS Rep. 2015, 12, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Mazzuca, P.; Caruso, A.; Caccuri, F. HIV-1 infection, microenvironment and endothelial cell dysfunction. New Microbiol. 2016, 39, 163–173. [Google Scholar]
- Porter, K.M.; Sutliff, R.L. HIV-1, reactive oxygen species, and vascular complications. Free Radic. Biol. Med. 2012, 53, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Kovalevich, J.; Langford, D. Neuronal toxicity in HIV CNS disease. Future Virol. 2012, 7, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Nath, A.; Haughey, N.J.; Jones, M.; Anderson, C.; Bell, J.E.; Geiger, J.D. Synergistic Neurotoxicity by Human Immunodeficiency Virus Proteins Tat and gp120: Protection by Memantine. Ann. Neurol. 2000, 47, 186–194. [Google Scholar] [CrossRef]
- Saylor, D.; Dickens, A.M.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-associated neurocognitive disorder-Pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016, 12, 234–248. [Google Scholar] [CrossRef]
- Van Marle, G.; Henry, S.; Todoruk, T.; Sullivan, A.; Silva, C.; Rourke, S.B.; Holden, J.; McArthur, J.C.; Gill, M.J.; Power, C. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: A neurotoxic role for IP-10. Virology 2004, 329, 302–318. [Google Scholar] [CrossRef] [Green Version]
- Nerlander, L.M.C.; Hoots, B.E.; Bradley, H.; Broz, D.; Thorson, A.; Paz-Bailey, G. HIV infection among MSM who inject methamphetamine in 8 US cities. Drug Alcohol Depend. 2018, 190, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Dai, Z.; Wen, S.; Bhandari, R. Risk factors associated with infection of blood-borne virus among people who used methamphetamine. BMC Infect. Dis. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Glick, S.N.; Burt, R.; Kummer, K.; Tinsley, J.; Banta-Green, C.J.; Golden, M.R. Increasing methamphetamine injection among non-MSM who inject drugs in King County, Washington. Drug Alcohol Depend. 2018, 182, 86–92. [Google Scholar] [CrossRef]
- Hoenigl, M.; Chaillon, A.; Moore, D.J.; Morris, S.R.; Smith, D.M.; Little, S.J. Clear links between starting methamphetamine and increasing sexual risk behavior: A cohort study among men who have sex with men. J. Acquir. Immune Defic. Syndr. 2016, 71, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Noroozi, M.; Higgs, P.; Noroozi, A.; Armoon, B.; Mousavi, B.; Alikhani, R.; Bazrafshan, M.R.; Astaneh, A.N.; Bayani, A.; Moghaddam, L.F. Methamphetamine use and HIV risk behavior among men who inject drugs: Causal inference using coarsened exact matching. Harm Reduct. J. 2020, 17, 1–7. [Google Scholar] [CrossRef]
- Feelemyer, J.; Arasteh, K.; Huong, D.T.; Oanh, K.T.H.; Khue, P.M.; Giang, H.T.; Thanh, N.T.T.; Moles, J.P.; Vinh, V.H.; Vallo, R.; et al. Associations between methamphetamine use and lack of viral suppression among a cohort of HIV-positive persons who inject drugs in Hai Phong, Vietnam. AIDS 2020, 34, 1875–1882. [Google Scholar] [CrossRef]
- Fulcher, J.A.; Javanbakht, M.; Shover, C.L.; Ragsdale, A.; Brookmeyer, R.; Shoptaw, S.; Gorbach, P.M. Comparative impact of methamphetamine and other drug use on viral suppression among sexual minority men on antiretroviral therapy. Drug Alcohol Depend. 2021, 221, 108622. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Lee, J.Y.; Fulcher, J.A.; Roach, M.E.; Dilworth, S.E.; Chahine, A.; Pallikkuth, S.; Fuchs, D.; Pahwa, S.; Carrico, A.W. Getting to the point: Methamphetamine injection is associated with biomarkers relevant to HIV pathogenesis. Drug Alcohol Depend. 2020, 213, 108133. [Google Scholar] [CrossRef]
- Sanchez, A.B.; Kaul, M. Neuronal stress and injury caused by HIV-1, cART and drug abuse: Converging contributions to HAND. Brain Sci. 2017, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Soontornniyomkij, V.; Umlauf, A.; Soontornniyomkij, B.; Batki, I.B.; Moore, D.J.; Masliah, E.; Achim, C.L. Lifetime methamphetamine dependence is associated with cerebral microgliosis in HIV-1 infected adults. J. Neurovirol. 2016, 22, 650. [Google Scholar] [CrossRef] [PubMed]
- Basova, L.; Lindsey, A.; McGovern, A.M.; Ellis, R.J.; Marcondes, M.C.G. Detection of h3k4me3 identifies neurohiv signatures, genomic effects of methamphetamine and addiction pathways in postmortem hiv+ brain specimens that are not amenable to transcriptome analysis. Viruses 2021, 13, 544. [Google Scholar] [CrossRef]
- Geng, J.; Wang, L.; Zhang, L.; Qin, C.; Song, Y.; Ma, Y.; Chen, Y.; Chen, S.; Wang, Y.; Zhang, Z.; et al. Blood-brain barrier disruption induced cognitive impairment is associated with increase of inflammatory cytokine. Front. Aging Neurosci. 2018, 10, 129. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Atluri, V.S.R.; Hidalgo, M.; Samikkannu, T.; Venkata Kurapati, K.R.; Jayant, R.D.; Sagar, V.; Nair, M.P.N. Effect of human immunodeficiency virus on blood-brain barrier integrity and function: An update. Front. Cell. Neurosci. 2015, 9, 1–10. [Google Scholar] [CrossRef]
- Louboutin, J.P.; Strayer, D.S. Blood-brain barrier abnormalities caused by HIV-1 gp120: Mechanistic and therapeutic implications. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef]
- Sajja, R.K.; Rahman, S.; Cucullo, L. Drugs of abuse and blood-brain barrier endothelial dysfunction: A focus on the role of oxidative stress. J. Cereb. Blood Flow Metab. 2016, 36, 539–554. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wang, Y.; Li, Q.; Zhong, Y.; Chen, L.; Du, Y.; He, J.; Liao, L.; Xiong, K.; Yi, C.X.; et al. The main molecular mechanisms underlying methamphetamine-induced neurotoxicity and implications for pharmacological treatment. Front. Mol. Neurosci. 2018, 11, 186. [Google Scholar] [CrossRef]
- Bell, A.H.; Miller, S.L.; Castillo-Melendez, M.; Malhotra, A. The Neurovascular Unit: Effects of Brain Insults During the Perinatal Period. Front. Neurosci. 2020, 13, 1452. [Google Scholar] [CrossRef] [PubMed]
- Burbelo, P.D.; Price, R.W.; Hagberg, L.; Hatano, H.; Spudich, S.; Deeks, S.G.; Gisslén, M. Anti-Human Immunodeficiency Virus Antibodies in the Cerebrospinal Fluid: Evidence of Early Treatment Impact on Central Nervous System Reservoir? J. Infect. Dis. 2018, 217, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, L.J.; Johnson, T.P.; Smith, B.R.; Reoma, L.B.; Santamaria, U.A.; Bachani, M.; Demarino, C.; Barclay, R.A.; Snow, J.; Sacktor, N.; et al. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS 2019, 33 (Suppl. 2), S145–S157. [Google Scholar] [CrossRef]
- Thaney, V.E.; Sanchez, A.B.; Fields, J.A.; Minassian, A.; Young, J.W.; Maung, R.; Kaul, M. Transgenic mice expressing HIV-1 envelope protein gp120 in the brain as an animal model in neuroAIDS research. J. Neurovirol. 2018, 24, 156–167. [Google Scholar] [CrossRef]
- Li, J.; Huang, J.; He, Y.; Wang, W.; Leung, C.-K.; Zhang, D.; Zhang, R.; Wang, S.; Li, Y.; Liu, L.; et al. The protective effect of gastrodin against the synergistic effect of HIV-Tat protein and METH on the blood–brain barrier via glucose transporter 1 and glucose transporter 3. Toxicol. Res. 2021, 10, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, R.; Wang, S.; Zhang, D.; Leung, C.K.; Yang, G.; Li, Y.; Liu, L.; Xu, Y.; Lin, S.; et al. Methamphetamine and HIV-Tat Protein Synergistically Induce Oxidative Stress and Blood-Brain Barrier Damage via Transient Receptor Potential Melastatin 2 Channel. Front. Pharmacol. 2021, 12, 619436. [Google Scholar] [CrossRef]
- Zeng, X.F.; Li, Q.; Li, J.; Wong, N.; Li, Z.; Huang, J.; Yang, G.; Sham, P.C.; Li, S.-B.; Lu, G. HIV-1 Tat and methamphetamine co-induced oxidative cellular injury is mitigated by N-acetylcysteine amide (NACA) through rectifying mTOR signaling. Toxicol. Lett. 2018, 299, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Baker, W.; Cambow, D.; Gogerty, D.; Leda, A.R.; Herlihy, B.; Pavlenko, D.; Van Den Nieuwenhuizen, S.; Toborek, M. Methamphetamine Enhances HIV-Induced Aberrant Proliferation of Neural Progenitor Cells via the FOXO3-Mediated Mechanism. Mol. Neurobiol. 2021, 1–16. [Google Scholar] [CrossRef]
- Yu, C.; Narasipura, S.D.; Richards, M.H.; Hu, X.T.; Yamamoto, B.; Al-Harthi, L. HIV and drug abuse mediate astrocyte senescence in a β-catenin-dependent manner leading to neuronal toxicity. Aging Cell 2017, 16, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, B.; Hu, X.; Li, Z.; Zhang, D.; Yang, G.; Dai, J.; Zeng, X. Protective effects of ginsenoside Rb1 against blood-brain barrier damage induced by human immunodeficiency virus-1 tat protein and methamphetamine in Sprague-Dawley rats. Am. J. Chin. Med. 2018, 46, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Hoefer, M.M.; Sanchez, A.B.; Maung, R.; de Rozieres, C.M.; Catalan, I.C.; Dowling, C.C.; Thaney, V.E.; Piña-Crespo, J.; Zhang, D.; Roberts, A.J.; et al. Combination of methamphetamine and HIV-1 gp120 causes distinct long-term alterations of behavior, gene expression, and injury in the central nervous system. Exp. Neurol. 2015, 263, 221–234. [Google Scholar] [CrossRef] [Green Version]
- De Guglielmo, G.; Fu, Y.; Chen, J.; Larrosa, E.; Hoang, I.; Kawamura, T.; Lorrai, I.; Zorman, B.; Bryant, J.; George, O.; et al. Increases in compulsivity, inflammation, and neural injury in HIV transgenic rats with escalated methamphetamine self-administration under extended-access conditions. Brain Res. 2020, 1726, 146502. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, L.; Shen, Q.; Bai, X.; Di, X. Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology. Behav. Neurol. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Ohene-Nyako, M.; Persons, A.L.; Napier, T.C. Region-specific changes in markers of neuroplasticity revealed in HIV-1 transgenic rats by low-dose methamphetamine. Brain Struct. Funct. 2018, 223, 3503–3513. [Google Scholar] [CrossRef]
- Najera, J.A.; Bustamante, E.A.; Bortell, N.; Morsey, B.; Fox, H.S.; Ravasi, T.; Marcondes, M.C.G. Methamphetamine abuse affects gene expression in brain-derived microglia of SIV-infected macaques to enhance inflammation and promote virus targets. BMC Immunol. 2016, 17, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Niu, M.; Morsey, B.; Lamberty, B.G.; Emanuel, K.; Yu, F.; León-Rivera, R.; Berman, J.W.; Gaskill, P.J.; Matt, S.M.; Ciborowski, P.S.; et al. Methamphetamine increases the proportion of siv-infected microglia/macrophages, alters metabolic pathways, and elevates cell death pathways: A single-cell analysis. Viruses 2020, 12, 1297. [Google Scholar] [CrossRef] [PubMed]
- Doulias, P.-T.; Nakamura, T.; Scott, H.; McKercher, S.R.; Sultan, A.; Deal, A.; Albertolle, M.; Ischiropoulos, H.; Lipton, S.A. TCA cycle metabolic compromise due to an aberrant S-nitrosoproteome in HIV-associated neurocognitive disorder with methamphetamine use. J. Neurovirol. 2021, 27, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Leibrand, C.R.; Palasuberniam, P.; Couraud, P.O.; Weksler, B.; Jahr, F.M.; McClay, J.L.; Hauser, K.F.; McRae, M.P. Effects of HIV-1 Tat and methamphetamine on blood-brain barrier integrity and function in vitro. Antimicrob. Agents Chemother. 2017, 61, e01307-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Fu, M.; Kumar, S.; Kumar, A. Methamphetamine potentiates HIV-1 gp120-mediated autophagy via beclin-1 and Atg5/7 as a pro-survival response in astrocytes. Cell Death Dis. 2016, 7, e2425. [Google Scholar] [CrossRef] [PubMed]
- Castellano, P.; Nwagbo, C.; Martinez, L.R.; Eugenin, E.A. Methamphetamine compromises gap junctional communication in astrocytes and neurons. J. Neurochem. 2016, 137, 561–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teodorof-Diedrich, C.; Spector, S.A. Human Immunodeficiency Virus Type 1 and Methamphetamine-Mediated Mitochondrial Damage and Neuronal Degeneration in Human Neurons. J. Virol. 2020, 94, e00924-20. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, W.; Tong, P.; Leung, C.K.; Yang, G.; Li, Z.; Li, N.; Sun, X.; Han, Y.; Lu, C.; et al. Autophagy Induction by HIV-Tat and Methamphetamine in Primary Midbrain Neuronal Cells of Tree Shrews via the mTOR Signaling and ATG5/ATG7 Pathway. Front. Neurosci. 2018, 12, 921. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, E.; Tu, G.; Liu, H.; Luo, J.; Xiong, H. Methamphetamine potentiates HIV-1gp120-induced microglial neurotoxic activity by enhancing microglial outward K+ current. Mol. Cell. Neurosci. 2017, 82, 167–175. [Google Scholar] [CrossRef]
- Fernandes, N.C.; Sriram, U.; Gofman, L.; Cenna, J.M.; Ramirez, S.H.; Potula, R. Methamphetamine alters microglial immune function through P2X7R signaling. J. Neuroinflamm. 2016, 13, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villabona-Rueda, A.; Erice, C.; Pardo, C.A.; Stins, M.F. The Evolving Concept of the Blood Brain Barrier (BBB): From a Single Static Barrier to a Heterogeneous and Dynamic Relay Center. Front. Cell. Neurosci. 2019, 13, 405. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef] [Green Version]
- Kakogiannos, N.; Ferrari, L.; Giampietro, C.; Scalise, A.A.; Maderna, C.; Ravà, M.; Taddei, A.; Lampugnani, M.G.; Pisati, F.; Malinverno, M.; et al. JAM-A acts via C/EBP-α to promote claudin-5 expression and enhance endothelial barrier function. Circ. Res. 2020, 127, 1056–1073. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Arima, M.; Nakao, S.; Yamaguchi, M.; Feng, H.; Fujii, Y.; Shibata, K.; Wada, I.; Kaizu, Y.; Ahmadieh, H.; Ishibashi, T.; et al. Claudin-5 redistribution induced by inflammation leads to anti-VEGF–resistant diabetic macular edema. Diabetes 2020, 69, 981–999. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Hanley, N.; Campbell, M. Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS 2019, 16, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ohene-Nyako, M.; Persons, A.L.; Napier, T.C. Hippocampal blood–brain barrier of methamphetamine self-administering HIV-1 transgenic rats. Eur. J. Neurosci. 2021, 53, 416–429. [Google Scholar] [CrossRef]
- Tauber, S.C.; Staszewski, O.; Prinz, M.; Weis, J.; Nolte, K.; Bunkowski, S.; Brück, W.; Nau, R. HIV encephalopathy: Glial activation and hippocampal neuronal apoptosis, but limited neural repair. HIV Med. 2016, 17, 143–151. [Google Scholar] [CrossRef]
- Choi, M.R.; Chun, J.W.; Kwak, S.M.; Bang, S.H.; Jin, Y.B.; Lee, Y.; Kim, H.N.; Chang, K.T.; Chai, Y.G.; Lee, S.R.; et al. Effects of acute and chronic methamphetamine administration on cynomolgus monkey hippocampus structure and cellular transcriptome. Toxicol. Appl. Pharmacol. 2018, 355, 68–79. [Google Scholar] [CrossRef]
- Weksler, B.; Romero, I.A.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melega, W.P.; Cho, A.K.; Harvey, D.; Laćan, G. Methamphetamine blood concentrations in human abusers: Application to pharmacokinetic modeling. Synapse 2007, 61, 216–220. [Google Scholar] [CrossRef]
- Fowler, J.S.; Volkow, N.D.; Logan, J.; Alexoff, D.; Telang, F.; Wang, G.-J.; Wong, C.; Ma, Y.; Kriplani, A.; Pradhan, K.; et al. Fast Uptake and Long-Lasting Binding of Methamphetamine in the Human Brain: Comparison with Cocaine. Neuroimage 2008, 43, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Fowler, J.S.; Wang, G.-J.; Shumay, E.; Telang, F.; Thanos, P.K.; Alexoff, D. Distribution and Pharmacokinetics of Methamphetamine in the Human Body: Clinical Implications. PLoS ONE 2010, 5, e15269. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Zhang, B.; Eum, S.Y.; Toborek, M. HIV-1 Tat triggers nuclear localization of ZO-1 via Rho signaling and cAMP response element-binding protein activation. J. Neurosci. 2012, 32, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; András, I.E.; Rha, G.B.; Hennig, B.; Toborek, M. PPARα and PPARγ protect against HIV-1-induced MMP-9 overexpression via caveolae-associated ERK and Akt signaling. FASEB J. 2011, 25, 3979–3988. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Kim, H.J.; Lim, B.; Wylegala, A.; Toborek, M. Methamphetamine-induced occludin endocytosis is mediated by the Arp2/3 complex-regulated actin rearrangement. J. Biol. Chem. 2013, 288, 33324–33334. [Google Scholar] [CrossRef] [Green Version]
- Toborek, M.; Seelbach, M.J.; Rashid, C.S.; András, I.E.; Chen, L.; Park, M.; Esser, K.A. Voluntary exercise protects against methamphetamine-induced oxidative stress in brain microvasculature and disruption of the blood-brain barrier. Mol. Neurodegener. 2013, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toborek, M.; Barger, S.W.; Mattson, M.P.; Barve, S.; McClain, C.J.; Hennig, B. Linoleic acid and TNF-α cross-amplify oxidative injury and dysfunction of endothelial cells. J. Lipid Res. 1996, 37, 123–135. [Google Scholar] [CrossRef]
- Toborek, M.; Barger, S.W.; Mattson, M.P.; McClain, C.J.; Hennig, B. Role of glutathione redox cycle in TNF-α-mediated endothelial cell dysfunction. Atherosclerosis 1995, 117, 179–188. [Google Scholar] [CrossRef]
- Patching, S.G. Glucose Transporters at the Blood-Brain Barrier: Function, Regulation and Gateways for Drug Delivery. Mol. Neurobiol. 2017, 54, 1046–1077. [Google Scholar] [CrossRef]
- Xia, M.; Ye, Z.; Shi, Y.; Zhou, L.; Hua, Y. Curcumin improves diabetes mellitus-associated cerebral infarction by increasing the expression of GLUT1 and GLUT3. Mol. Med. Rep. 2018, 17, 1963–1969. [Google Scholar] [CrossRef]
- Abdul Muneer, P.M.; Alikunju, S.; Szlachetka, A.M.; Murrin, L.C.; Haorah, J. Impairment of brain endothelial glucose transporter by methamphetamine causes blood-brain barrier dysfunction. Mol. Neurodegener. 2011, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Alam, C.; Whyte-Allman, S.K.; Omeragic, A.; Bendayan, R. Role and modulation of drug transporters in HIV-1 therapy. Adv. Drug Deliv. Rev. 2016, 103, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Dickens, A.M.; Yoo, S.W.; Chin, A.C.; Xu, J.; Johnson, T.P.; Trout, A.L.; Hauser, K.F.; Haughey, N.J. Chronic low-level expression of HIV-1 Tat promotes a neurodegenerative phenotype with aging. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Toborek, M.; Lee, Y.W.; Pu, H.; Malecki, A.; Flora, G.; Garrido, R.; Hennig, B.; Bauer, H.C.; Nath, A. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J. Neurochem. 2003, 84, 169–179. [Google Scholar] [CrossRef]
- Namyen, J.; Permpoonputtana, K.; Nopparat, C.; Tocharus, J.; Tocharus, C.; Govitrapong, P. Protective Effects of Melatonin on Methamphetamine-Induced Blood–Brain Barrier Dysfunction in Rat Model. Neurotox. Res. 2020, 37, 640–660. [Google Scholar] [CrossRef]
- Herland, A.; Maoz, B.M.; FitzGerald, E.A.; Grevesse, T.; Vidoudez, C.; Sheehy, S.P.; Budnik, N.; Dauth, S.; Mannix, R.; Budnik, B.; et al. Proteomic and Metabolomic Characterization of Human Neurovascular Unit Cells in Response to Methamphetamine. Adv. Biosyst. 2020, 4, 1900230. [Google Scholar] [CrossRef] [PubMed]
- Alawieyah Syed Mortadza, S.; Sim, J.A.; Neubrand, V.E.; Jiang, L.H. A critical role of TRPM2 channel in Aβ42-induced microglial activation and generation of tumor necrosis factor-α. Glia 2018, 66, 562–575. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Negro, R.; Wu, H. TRPM2, linking oxidative stress and Ca2+ permeation to NLRP3 inflammasome activation. Curr. Opin. Immunol. 2020, 62, 131–135. [Google Scholar] [CrossRef]
- Jiang, Q.; Gao, Y.; Wang, C.; Tao, R.; Wu, Y.; Zhan, K.; Liao, M.; Lu, N.; Lu, Y.; Wilcox, C.S.; et al. Nitration of TRPM2 as a Molecular Switch Induces Autophagy during Brain Pericyte Injury. Antioxid. Redox Signal. 2017, 27, 1297–1316. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Dual roles of astrocyte-derived factors in regulation of blood-brain barrier function after brain damage. Int. J. Mol. Sci. 2019, 20, 571. [Google Scholar] [CrossRef] [Green Version]
- Guérit, S.; Fidan, E.; Macas, J.; Czupalla, C.J.; Figueiredo, R.; Vijikumar, A.; Yalcin, B.H.; Thom, S.; Winter, P.; Gerhardt, H.; et al. Astrocyte-derived Wnt growth factors are required for endothelial blood-brain barrier maintenance. Prog. Neurobiol. 2021, 199, 101937. [Google Scholar] [CrossRef] [PubMed]
- Heithoff, B.P.; George, K.K.; Phares, A.N.; Zuidhoek, I.A.; Munoz-Ballester, C.; Robel, S. Astrocytes are necessary for blood–brain barrier maintenance in the adult mouse brain. Glia 2021, 69, 436–472. [Google Scholar] [CrossRef]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayant, R.D.; Tiwari, S.; Atluri, V.; Kaushik, A.; Tomitaka, A.; Yndart, A.; Colon-Perez, L.; Febo, M.; Nair, M. Multifunctional Nanotherapeutics for the Treatment of neuroAIDS in Drug Abusers. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohannon, D.G.; Ko, A.; Filipowicz, A.R.; Kuroda, M.J.; Kim, W.K. Dysregulation of sonic hedgehog pathway and pericytes in the brain after lentiviral infection. J. Neuroinflamm. 2019, 16, 1–13. [Google Scholar] [CrossRef]
- Bohannon, D.G.; Okhravi, H.R.; Kim, J.; Kuroda, M.J.; Didier, E.S.; Kim, W.K. A subtype of cerebrovascular pericytes is associated with blood-brain barrier disruption that develops during normal aging and simian immunodeficiency virus infection. Neurobiol. Aging 2020, 96, 128–136. [Google Scholar] [CrossRef]
- Cho, H.J.; Kuo, A.M.S.; Bertrand, L.; Toborek, M. HIV alters gap junction-mediated intercellular communication in human brain pericytes. Front. Mol. Neurosci. 2017, 10, 410. [Google Scholar] [CrossRef] [Green Version]
- Castro, V.; Bertrand, L.; Luethen, M.; Dabrowski, S.; Lombardi, J.; Morgan, L.; Sharova, N.; Stevenson, M.; Blasig, I.E.; Toborek, M. Occludin controls HIV transcription in brain pericytes via regulation of SIRT-1 activation. FASEB J. 2016, 30, 1234–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torices, S.; Roberts, S.A.; Park, M.; Malhotra, A.; Toborek, M. Occludin, caveolin-1, and Alix form a multi-protein complex and regulate HIV-1 infection of brain pericytes. FASEB J. 2020, 34, 16319–16332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Bai, Y.; Chao, J.; Hu, G.; Chen, X.; Yao, H. Involvement of PUMA in pericyte migration induced by methamphetamine. Exp. Cell Res. 2017, 356, 28–39. [Google Scholar] [CrossRef]
- Piekna-Przybylska, D.; Nagumotu, K.; Reid, D.M.; Maggirwar, S.B. HIV-1 infection renders brain vascular pericytes susceptible to the extracellular glutamate. J. Neurovirol. 2019, 25, 114–126. [Google Scholar] [CrossRef]
- Harms, R.; Morsey, B.; Boyer, C.W.; Fox, H.S.; Sarvetnick, N. Methamphetamine Administration Targets Multiple Immune Subsets and Induces Phenotypic Alterations Suggestive of Immunosuppression. PLoS ONE 2012, 7, e49897. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Wang, M.; Liang, B.; Shi, Y.; Su, Q.; Chen, H.; Huang, J.; Su, J.; Pan, P.; Li, Y.; et al. In vivo effects of methamphetamine on HIV-1 replication: A population-based study. Drug Alcohol Depend. 2016, 159, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Carrico, A.W.; Cherenack, E.M.; Roach, M.E.; Riley, E.D.; Oni, O.; DIlworth, S.E.; Shoptaw, S.; Hunt, P.; Roy, S.; Pallikkuth, S.; et al. Substance-associated elevations in monocyte activation among methamphetamine users with treated HIV infection. AIDS 2018, 32, 767–771. [Google Scholar] [CrossRef]
- Liang, H.; Wang, X.; Chen, H.; Song, L.; Ye, L.; Wang, S.H.; Wang, Y.J.; Zhou, L.; Ho, W.Z. Methamphetamine enhances HIV infection of macrophages. Am. J. Pathol. 2008, 172, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.S.; Prasad, A.; Groopman, J.E. Methamphetamine Enhances HIV-1 Replication in CD4+ T-Cells via a Novel IL-1β Auto-Regulatory Loop. Front. Immunol. 2020, 11, 136. [Google Scholar] [CrossRef]
- Prasad, A.; Kulkarni, R.; Shrivastava, A.; Jiang, S.; Lawson, K.; Groopman, J.E. Methamphetamine functions as a novel CD4+ T-cell activator via the sigma-1 receptor to enhance HIV-1 infection. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, M.C.G.; Flynn, C.; Watry, D.D.; Zandonatti, M.; Fox, H.S. Methamphetamine increases brain viral load and activates natural killer cells in simian immunodeficiency virus-infected monkeys. Am. J. Pathol. 2010, 177, 355–361. [Google Scholar] [CrossRef]
- Basova, L.; Najera, J.A.; Bortell, N.; Wang, D.; Moya, R.; Lindsey, A.; Semenova, S.; Ellis, R.J.; Marcondes, M.C.G. Dopamine and its receptors play a role in the modulation of CCR5 expression in innate immune cells following exposure to Methamphetamine: Implications to HIV infection. PLoS ONE 2018, 13, e0199861. [Google Scholar] [CrossRef] [PubMed]
- Brilha, S.; Ong, C.W.M.; Weksler, B.; Romero, N.; Couraud, P.O.; Friedland, J.S. Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Rosell, A.; Cuadrado, E.; Ortega-Aznar, A.; Hernández-Guillamon, M.; Lo, E.H.; Montaner, J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 2008, 39, 1121–1126. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Anderson, P.; Durbeej, M.; Van Rooijen, N.; Ivars, F.; Opdenakker, G.; Sorokin, L.M. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med. 2006, 203, 1007–1016. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Chen, L.; Zhang, B.; Park, M.; Toborek, M. PPAR agonist-mediated protection against HIV Tat-induced cerebrovascular toxicity is enhanced in MMP-9-deficient mice. J. Cereb. Blood Flow Metab. 2014, 34, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Pu, H.; Hayashi, K.; Andras, I.E.; Eum, S.Y.; Hennig, B.; Toborek, M. Limited role of COX-2 in HIV Tat-induced alterations of tight junction protein expression and disruption of the blood-brain barrier. Brain Res. 2007, 1184, 333–344. [Google Scholar] [CrossRef]
- Northrop, N.A.; Yamamoto, B.K. Persistent neuroinflammatory effects of serial exposure to stress and methamphetamine on the blood-brain barrier. J. Neuroimmune Pharmacol. 2012, 7, 951–968. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.T.; Gilpin, K.; Adnan, A. Effects of Focal Axonal Swelling Level on the Action Potential Signal Transmission. J. Comput. Neurosci. 2020, 48, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Byun, N.; Delpire, E. Axonal and periaxonal swelling precede peripheral neurodegeneration in KCC3 knockout mice. Neurobiol. Dis. 2007, 28, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Carbonell, D.; Ye, F.; Ramanath, N.; Garcia-Mesa, Y.; Knapp, P.E.; Hauser, K.F.; Karn, J. Cross-talk between microglia and neurons regulates HIV latency. PLoS Pathog. 2019, 15, e1008249. [Google Scholar] [CrossRef] [Green Version]
- Mediouni, S.; Garibaldi Marcondes, M.C.; Miller, C.; McLaughlin, J.P.; Valente, S.T. The cross-talk of HIV-1 Tat and methamphetamine in HIV-associated neurocognitive disorders. Front. Microbiol. 2015, 6, 1164. [Google Scholar] [CrossRef] [Green Version]
- Rangaraju, V.; Lauterbach, M.; Schuman, E.M. Spatially Stable Mitochondrial Compartments Fuel Local Translation during Plasticity. Cell 2019, 176, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallianpur, K.J.; Walker, M.; Gerschenson, M.; Shikuma, C.M.; Gangcuangco, L.M.A.; Kohorn, L.; Libutti, D.E.; Nir, T.M.; Jahanshad, N.; Thompson, P.M.; et al. Systemic Mitochondrial Oxidative Phosphorylation Protein Levels Correlate with Neuroimaging Measures in Chronically HIV-Infected Individuals. AIDS Res. Hum. Retrovir. 2020, 36, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Iudicello, J.E.; Lin, J.; Ellis, R.J.; Morgan, E.; Okwuegbuna, O.; Cookson, D.; Karris, M.; Saloner, R.; Heaton, R.; et al. Telomere length is associated with HIV infection, methamphetamine use, inflammation, and comorbid disease risk. Drug Alcohol Depend. 2021, 221, 108639. [Google Scholar] [CrossRef]
- Fields, J.A.; Serger, E.; Campos, S.; Divakaruni, A.S.; Kim, C.; Smith, K.; Trejo, M.; Adame, A.; Spencer, B.; Rockenstein, E.; et al. HIV alters neuronal mitochondrial fission/fusion in the brain during HIV-associated neurocognitive disorders. Neurobiol. Dis. 2016, 86, 154–169. [Google Scholar] [CrossRef] [Green Version]
- Avdoshina, V.; Fields, J.A.; Castellano, P.; Dedoni, S.; Palchik, G.; Trejo, M.; Adame, A.; Rockenstein, E.; Eugenin, E.; Masliah, E.; et al. The HIV Protein gp120 Alters Mitochondrial Dynamics in Neurons. Neurotox. Res. 2016, 29, 583–593. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Murrin, L.C.; Zheng, J.C. Mitochondrial fragmentation is involved in methamphetamine-induced cell death in rat hippocampal neural progenitor cells. PLoS ONE 2009, 4, e5546. [Google Scholar] [CrossRef] [Green Version]
- Skowronska, M.; McDonald, M.; Velichkovska, M.; Rachel Leda, A.; Park, M.; Toborek, M. Methamphetamine increases HIV infectivity in neural progenitor cells. J. Biol. Chem. 2018, 293, 296–311. [Google Scholar] [CrossRef] [Green Version]
- Pozhilenkova, E.A.; Lopatina, O.L.; Komleva, Y.K.; Salmin, V.V.; Salmina, A.B. Blood-brain barrier-supported neurogenesis in healthy and diseased brain. Rev. Neurosci. 2017, 28, 397–415. [Google Scholar] [CrossRef]
- Nickoloff-Bybel, E.A.; Calderon, T.M.; Gaskill, P.J.; Berman, J.W. HIV Neuropathogenesis in the Presence of a Disrupted Dopamine System. J. Neuroimmune Pharmacol. 2020, 15, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Tu, G.; Ying, L.; Ye, L.; Zhao, J.; Liu, N.; Li, J.; Liu, Y.; Zhu, M.; Wu, Y.; Xiao, B.; et al. Dopamine D1 and D2 Receptors Differentially Regulate Rac1 and Cdc42 Signaling in the Nucleus Accumbens to Modulate Behavioral and Structural Plasticity After Repeated Methamphetamine Treatment. Biol. Psychiatry 2019, 86, 820–835. [Google Scholar] [CrossRef] [PubMed]
- Hasbi, A.; Sivasubramanian, M.; Milenkovic, M.; Komarek, K.; Madras, B.K.; George, S.R. Dopamine D1-D2 receptor heteromer expression in key brain regions of rat and higher species: Upregulation in rat striatum after cocaine administration. Neurobiol. Dis. 2020, 143, 105017. [Google Scholar] [CrossRef] [PubMed]
- Baek, E.J.; Kim, H.; Basova, L.A.; Rosander, A.; Kesby, J.P.; Semenova, S.; Marcondes, M.C.G. Sex differences and Tat expression affect dopaminergic receptor expression and response to antioxidant treatment in methamphetamine-sensitized HIV Tat transgenic mice. Neuropharmacology 2020, 178, 108245. [Google Scholar] [CrossRef]
- Ashok, A.H.; Mizuno, Y.; Volkow, N.D.; Howes, O.D. Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine a systematic review and meta-analysis. JAMA Psychiatry 2017, 74, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Proebstl, L.; Kamp, F.; Manz, K.; Krause, D.; Adorjan, K.; Pogarell, O.; Koller, G.; Soyka, M.; Falkai, P.; Kambeitz, J. Effects of stimulant drug use on the dopaminergic system: A systematic review and meta-analysis of in vivo neuroimaging studies. Eur. Psychiatry 2019, 59, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Borgmann, K.; Ghorpade, A. Methamphetamine augments concurrent astrocyte mitochondrial stress, oxidative burden, and antioxidant capacity: Tipping the balance in HIV-associated neurodegeneration. Neurotox. Res. 2018, 33, 433–447. [Google Scholar] [CrossRef]
- Martínez-Cué, C.; Rueda, N. Cellular Senescence in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 956–965. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cisneros, I.E. Methamphetamine Activates Trace Amine Associated Receptor 1 to Regulate Astrocyte Excitatory Amino Acid Transporter-2 via Differential CREB Phosphorylation During HIV-Associated Neurocognitive Disorders. Front. Neurol. 2020, 11, 1552. [Google Scholar] [CrossRef]
- Walker, K.A.; Brown, G.G. HIV-associated executive dysfunction in the era of modern antiretroviral therapy: A systematic review and meta-analysis. J. Clin. Exp. Neuropsychol. 2018, 40, 357–376. [Google Scholar] [CrossRef]
- Kesby, J.P.; Fields, J.A.; Chang, A.; Coban, H.; Achim, C.L.; Semenova, S. Effects of HIV-1 TAT protein and methamphetamine exposure on visual discrimination and executive function in mice. Behav. Brain Res. 2018, 349, 73–79. [Google Scholar] [CrossRef]
- Nookala, A.R.; Schwartz, D.C.; Chaudhari, N.S.; Glazyrin, A.; Stephens, E.B.; Berman, N.E.J.; Kumar, A. Methamphetamine augment HIV-1 Tat mediated memory deficits by altering the expression of synaptic proteins and neurotrophic factors. Brain. Behav. Immun. 2018, 71, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Pocuca, N.; Young, J.W.; MacQueen, D.A.; Letendre, S.; Heaton, R.K.; Geyer, M.A.; Perry, W.; Grant, I.; Minassian, A. Sustained attention and vigilance deficits associated with HIV and a history of methamphetamine dependence. Drug Alcohol Depend. 2020, 215, 108245. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.J.; Young, J.W.; Milienne-Petiot, M.; Deben, D.S.; Heaton, R.K.; Letendre, S.; Grelotti, D.J.; Perry, W.; Grant, I.; Minassian, A. Both HIV and Tat expression decrease prepulse inhibition with further impairment by methamphetamine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110089. [Google Scholar] [CrossRef] [PubMed]
- Minassian, A.; Henry, B.L.; Iudicello, J.E.; Morgan, E.E.; Letendre, S.L.; Heaton, R.K.; Perry, W. Everyday functional ability in HIV and methamphetamine dependence. Drug Alcohol Depend. 2017, 175, 60–66. [Google Scholar] [CrossRef]
- Paolillo, E.W.; Saloner, R.; Montoya, J.L.; Campbell, L.M.; Pasipanodya, E.C.; Iudicello, J.E.; Moore, R.C.; Letendre, S.L.; Jeste, D.V.; Moore, D.J. Frailty in comorbid HIV and lifetime methamphetamine use disorder: Associations with neurocognitive and everyday functioning. AIDS Res. Hum. Retrovir. 2019, 35, 1044–1053. [Google Scholar] [CrossRef] [Green Version]
- Sun-Suslow, N.; Saloner, R.; Serrano, V.; Umlauf, A.; Morgan, E.E.; Ellis, R.J.; Letendre, S.; Grant, I.; Heaton, R.K. Lifetime Methamphetamine Use Disorder and Reported Sleep Quality in Adults Living with HIV. AIDS Behav. 2020, 24, 3071–3082. [Google Scholar] [CrossRef]
- Saloner, R.; Cherner, M.; Iudicello, J.E.; Heaton, R.K.; Letendre, S.L.; Ellis, R.J. Cerebrospinal Fluid Norepinephrine and Neurocognition in HIV and Methamphetamine Dependence. J. Acquir. Immune Defic. Syndr. 2020, 85, e12–e22. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, M.; Shoptaw, S.; Ragsdale, A.; Brookmeyer, R.; Bolan, R.; Gorbach, P.M. Depressive symptoms and substance use: Changes overtime among a cohort of HIV-positive and HIV-negative MSM. Drug Alcohol Depend. 2020, 207, 107770. [Google Scholar] [CrossRef]
- Kesby, J.P.; Markou, A.; Semenova, S. The effects of HIV-1 regulatory TAT protein expression on brain reward function, response to psychostimulants and delay-dependent memory in mice. Neuropharmacology 2016, 109, 205–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesby, J.P.; Chang, A.; Najera, J.A.; Marcondes, M.C.G.; Semenova, S. Brain Reward Function after Chronic and Binge Methamphetamine Regimens in Mice Expressing the HIV-1 TAT Protein. Curr. HIV Res. 2019, 17, 126–133. [Google Scholar] [CrossRef]
- Zoetmulder, M.; Biernat, H.B.; Nikolic, M.; Korbo, L.; Friberg, L.; Jennum, P.J. Prepulse inhibition is associated with attention, processing speed, and 123I-FP-CIT SPECT in Parkinson’s Disease. J. Parkinson’s Dis. 2014, 4, 77–87. [Google Scholar] [CrossRef]
- Fjell, A.M.; Sørensen, Ø.; Amlien, I.K.; Bartrés-Faz, D.; Bros, D.M.; Buchmann, N.; Demuth, I.; Drevon, C.A.; Düzel, S.; Ebmeier, K.P.; et al. Self-reported sleep relates to hippocampal atrophy across the adult lifespan: Results from the lifebrain consortium. Sleep 2020, 43, zsz280. [Google Scholar] [CrossRef] [Green Version]
- Basova, L.V.; Kesby, J.P.; Kaul, M.; Semenova, S.; Marcondes, M.C.G. Systems biology analysis of the antagonizing effects of HIV-1 Tat expression in the brain over transcriptional changes caused by methamphetamine sensitization. Viruses 2020, 12, 426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffin, P.O.; Santos, G.M.; Hern, J.; Vittinghoff, E.; Walker, J.E.; Matheson, T.; Santos, D.; Colfax, G.; Batki, S.L. Effects of Mirtazapine for Methamphetamine Use Disorder among Cisgender Men and Transgender Women Who Have Sex with Men: A Placebo-Controlled Randomized Clinical Trial. JAMA Psychiatry 2020, 77, 246–255. [Google Scholar] [CrossRef]
- Moore, D.J.; Pasipanodya, E.C.; Umlauf, A.; Rooney, A.S.; Gouaux, B.; Depp, C.A.; Atkinson, J.H.; Montoya, J.L. Individualized texting for adherence building (iTAB) for methamphetamine users living with HIV: A pilot randomized clinical trial. Drug Alcohol Depend. 2018, 189, 154–160. [Google Scholar] [CrossRef]
- Jayant, R.D.; Atluri, V.S.R.; Tiwari, S.; Pilakka-Kanthikeel, S.; Kaushik, A.; Yndart, A.; Nair, M. Novel nanoformulation to mitigate co-effects of drugs of abuse and HIV-1 infection: Towards the treatment of NeuroAIDS. J. Neurovirol. 2017, 23, 603–614. [Google Scholar] [CrossRef] [PubMed]
- McMahan, V.M.; Frank, N.; Buckler, S.; Violette, L.R.; Baeten, J.M.; Banta-Green, C.J.; Barnabas, R.V.; Simoni, J.; Stekler, J.D. Protocol development for HMU! (HIV prevention for methamphetamine users), a study of peer navigation and text messaging to promote pre-exposure prophylaxis adherence and persistence among people who use methamphetamine: Qualitative focus group and intervi. JMIR Form. Res. 2020, 4, e18118. [Google Scholar] [CrossRef]
- Pasipanodya, E.C.; Kohli, M.; Kohli, M.; Fisher, C.B.; Moore, D.J.; Curtis, B. Perceived risks and amelioration of harm in research using mobile technology to support antiretroviral therapy adherence in the context of methamphetamine use: A focus group study among minorities living with HIV. Harm Reduct. J. 2020, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reback, C.J.; Fletcher, J.B.; Swendeman, D.A.; Metzner, M. Theory-Based Text-Messaging to Reduce Methamphetamine Use and HIV Sexual Risk Behaviors Among Men Who Have Sex with Men: Automated Unidirectional Delivery Outperforms Bidirectional Peer Interactive Delivery. AIDS Behav. 2019, 23, 37–47. [Google Scholar] [CrossRef] [PubMed]



| Study and Year | Experimental Model | Viral Inoculum Dose/Route | METH Dosing Regimen | Synergistic Effects on NVU |
|---|---|---|---|---|
| Combined In Vivo and In Vitro Studies | ||||
| Huang et al., 2021 [34] | Tree shrews; HCMEC/D3 cell line (human) | Tat (100 ng) by tail i.v. injection; 25 to 200 nM of Tat | 8 mg/kg i.p. for 10 consecutive days; 0.05 to 2.0 mM for 24 h | Enhanced BBB permeability due to alterations in TRPM2 channels and TJ protein expression both in vivo and in vitro |
| Li et al., 2021 [35] | Tree shrews; HCMEC/D3 cell line (human) | Tat (100 ng) by tail i.v. injection; 100 nM of Tat | 8 mg/kg i.p. for 10 consecutive days; 500 μM for 24 h | Decreased expression of TJ proteins and increased BBB permeability both in vivo and in vitro; Downregulation of GLUT1 and GLUT3 protein expression both in vivo and in vitro |
| Zeng et al., 2018 [36] | Rats; SH-SY5Y neuroblastoma cell line (human) | Tat (50 ng/kg) by tail i.v. injection; 50 and 100 nM of Tat | 10 mg/kg i.p. for 7 consecutive days; 1 and 2 mM for 24 h | Exacerbation of oxidative stress both in vivo and in vitro |
| Park et al., 2021 [37] | C57BL/6 mice; human primary neural progenitor cells | EcoHIV (1 μg of p24) via left internal carotid artery injection; HIV-1 NL4-3 (60 ng/mL of p24) | Escalating dose regimen for 6 days: 1.0–4.0 mg/kg i.p.; 100 μM for 24 h | Enhanced neural progenitor cell proliferation both in vivo and in vitro |
| Yu et al., 2017 [38] | HIV-1 transgenic rats; primary human fetal astrocytes | N/A; HIV-1 BaL (10 ng or 20 ng of p24) | 10 mg/kg i.p. every 2 h for 4 days; 10, 30, 100, 300 and 1000 μM daily for 5 days | Induction of astrocyte senescence both in vivo and in vitro |
| In Vivo Studies | ||||
| Li et al., 2018 [39] | Sprague-Dawley rats | Tat (50 ng) i.c.v. | 10 mg/kg i.p. for 7 consecutive days | Decreased expression of TJ proteins and increased BBB permeability; Exacerbation of oxidative stress and neuronal damage |
| Hoefer et al., 2015 [40] | HIV-1 gp120 transgenic mice | N/A | Escalating dose multiple-binge regimen for 25 days: 0.1–6.0 mg/kg s.c. | Reduction in post-tetanic potentiation in hippocampal slices; Decreased dendritic spine density |
| de Guglielmo et al., 2015 [41] | HIV-1 transgenic rats | N/A | Escalating dose multiple-binge regimen for 15 consecutive sessions: 0.5 mg/kg/0.1 mL 6 h/day i.v. | Gene expression changes indicative of an increase in neuronal damage and impaired aerobic glucose metabolism in the medial prefrontal cortex |
| Ohene-Nyako et al., 2018 [42] | HIV-1 transgenic rats | N/A | 0.02–0.04 mg/kg/0.05 mL i.v. infusion 2 h/day for 21 days | Upregulation of DRD1 and deltaFosB expression in the nucleus accumbens |
| Baek et al., 2020 [43] | Doxycycline-inducible HIV-1 Tat transgenic mice | N/A | 2 mg/kg i.p. once a day for 7 days (acquisition phase); 1 mg/kg (challenge phase) | Reduction in DRD2 and DRD5 mRNA levels in the striatum |
| Najera et al., 2016 [44] | Rhesus macaques | SIVmac251 i.v. (infectious dose was not reported) | Escalating dose regimen for 23 weeks with a final dose of 2.5 mg/kg i.m. | Upregulation of genes encoding proteins involved in DNA damage and senescence in microglia |
| Niu et al., 2020 [45] | Rhesus macaques | SIVmac251 i.v. (infectious dose was not reported) | Escalating dose regimen over a month-long period: 0.1–2.5 mg/kg i.m. | Upregulation of genes encoding proteins involved in cell death pathways and deficiencies in the BDNF-signaling pathway in brain microglia/macrophages |
| Postmortem Human Brain Ex Vivo Studies | ||||
| Soontornniyomkij et al., 2016 [22] | Human postmortem brain samples | N/A | Lifetime METH dependence | Focal cerebral microgliosis |
| Doulias et al., 2021 [46] | Human postmortem brain samples | N/A | Duration of METH use was not reported | Increase in S-nitrosylation of tricarboxylic acid enzymes |
| In Vitro Studies | ||||
| Patel et al., 2017 [47] | HCMEC/D3 cell line (human) | Tat (100 nM) | 10 μM for 24 h | Reduced ZO-1 TJ protein expression (in line with in vivo studies [34,35,39]); Increased rhodamine 123 accumulation |
| Cao et al., 2016 [48] | Simian virus 40 (SV40)-transformed astrocyte cell line (human) | gp120 (400 pM) | 500 μM for 24 h | Autophagy initiation |
| Castellano et al., 2016 [49] | Human primary mixed cultures of neurons and astrocytes | HIV-1 ADA (infectious dose was not reported) | 1 and 10 μM for 7,14 and 21 days | Enhancement of apoptosis |
| Teodorof-Diedrich et al., 2020 [50] | Human primary neurons | gp120, Tat or gp120/Tat (100 ng/mL) | 300 μM for 24 h | DRP1-dependent mitochondrial fragmentation; Neurite length reduction (in line with in vivo study [50]) |
| Li et al., 2018 [51] | Tree shrew primary midbrain neuronal cells | Tat (50 nM and 100 nM) | 0.1–0.5 mM at varying time periods | Autophagy initiation |
| Liu et al., 2017 [52] | Cultured rat microglial cells | gp120 (0.1, 0.5 and 1.5 nM) | 2, 20, and 200 µM for 24 h | Induced KV1.3 potassium channel- mediated microglial neurotoxicity; Increased caspase-3/7 activity in microglia (in line with in vivo studies [52,53]) |
| Study and Year | Study Design | Experimental Model | Study Outcomes |
|---|---|---|---|
| Human studies | |||
| Pocuca et al., 2020 [137] | Cross-sectional | 205 adults (67 HIV-/METH-, 36 HIV-/METH+, 49 HIV+/METH-, and 53 HIV+/METH+) | METH, but not HIV-1, was associated with sustained attention and vigilance deficits. |
| Walter et al., 2021 [138] | Cross-sectional | 205 adults (69 HIV-/METH-, 40 HIV-/METH+, 52 HIV+/METH-, and 44 HIV+/METH+) | Prepulse inhibition was most decreased in people with HIV-1 and a history of METH dependence. |
| Minassian et al., 2017 [139] | Cross-sectional | 172 adults (49 HIV-/METH-, 48 HIV-/METH+, 37 HIV+/METH-, and 38 HIV+/METH+) | Additive effects of HIV-1 and METH were not observed for everyday functioning. |
| Paolillo et al., 2019 [140] | Cross-sectional | 210 adults (92 HIV-/METH-, 75 HIV+/METH-, and 43 HIV+/METH+) | Persons with comorbid HIV-1 and METH use disorder had higher frailty index scores than both HIV-/MA- and HIV+/MA- participants. Additional models linked higher frailty index scores to worse global neurocognition and greater likelihood of everyday functioning dependence among the HIV+/METH+ group. |
| Sun-Suslow et al., 2020 [141] | Cross-sectional | 313 adults (72 HIV-/METH-, 16 HIV-/METH+, 141 HIV+/METH-, and 84 HIV+/METH+) | HIV+/METH+ individuals endorsed significantly more problematic sleep than HIV+ and HIV-/METH- individuals. Poorer reported sleep quality among HIV+/METH+ was also associated with multiple adverse functional outcomes including greater objective cognitive impairment. |
| Saloner et al., 2020 [142] | Cross-sectional | 125 adults (23 HIV-/METH-, 35 HIV-/METH+, 22 HIV+/METH-, and 45 HIV+/METH+) | Prevalence of lifetime major depression disorder was higher in HIV+/METH+ compared with the other groups. |
| Javanbakht et al., 2020 [143] | Longitudinal | 534 men (267 HIV+ and 267 HIV-); METH use was not individually reported | Frequent METH use, but not HIV status, was associated with persistence of depressive symptoms. |
| Rodent Studies | |||
| Kesby et al., 2018 [135] | Cross-sectional | Doxycycline-inducible HIV-1 Tat transgenic mouse model | The combination of Tat expression and METH exposure did not induce significant learning deficits but did increase perseverations at the initiation of reversal learning suggesting slight impairments in executive function. |
| Nookala et al., 2018 [136] | Cross-sectional | Doxycycline-inducible HIV-1 Tat transgenic mouse model | Administration of METH to HIV-1 Tat transgenic mice exacerbated the deficits in spatial learning and memory characterized by decreased spontaneous alternations in Y maze and increased latency time to reach the escape platform in the Morris water maze. |
| Walter et al., 2021 [138] | Cross-sectional | Doxycycline-inducible HIV-1 Tat transgenic mouse model | Chronic METH treatment and Tat expression did not interact to affect prepulse inhibition in mice. |
| Kesby et al., 2016 [144] | Cross-sectional | Doxycycline-inducible HIV-1 Tat transgenic mouse model | Tat expression in mice led to reward deficits, a core symptom of depression, and a greater sensitivity to METH-induced reward enhancement. |
| Kesby et al., 2019 [145] | Cross-sectional | Doxycycline-inducible HIV-1 Tat transgenic mouse model | Longer-term Tat expression, or its induction at earlier stages of METH exposure, was more consequential at inducing behavioral and neurochemical effects. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattakhov, N.; Torices, S.; Stangis, M.; Park, M.; Toborek, M. Synergistic Impairment of the Neurovascular Unit by HIV-1 Infection and Methamphetamine Use: Implications for HIV-1-Associated Neurocognitive Disorders. Viruses 2021, 13, 1883. https://doi.org/10.3390/v13091883
Fattakhov N, Torices S, Stangis M, Park M, Toborek M. Synergistic Impairment of the Neurovascular Unit by HIV-1 Infection and Methamphetamine Use: Implications for HIV-1-Associated Neurocognitive Disorders. Viruses. 2021; 13(9):1883. https://doi.org/10.3390/v13091883
Chicago/Turabian StyleFattakhov, Nikolai, Silvia Torices, Michael Stangis, Minseon Park, and Michal Toborek. 2021. "Synergistic Impairment of the Neurovascular Unit by HIV-1 Infection and Methamphetamine Use: Implications for HIV-1-Associated Neurocognitive Disorders" Viruses 13, no. 9: 1883. https://doi.org/10.3390/v13091883
APA StyleFattakhov, N., Torices, S., Stangis, M., Park, M., & Toborek, M. (2021). Synergistic Impairment of the Neurovascular Unit by HIV-1 Infection and Methamphetamine Use: Implications for HIV-1-Associated Neurocognitive Disorders. Viruses, 13(9), 1883. https://doi.org/10.3390/v13091883