Pseudo-Mannosylated DC-SIGN Ligands as Potential Adjuvants for HIV Vaccines
Abstract
:1. Introduction
2. Results and Discussion
2.1.Inhibition of HIV-1 Infection of Human Cervical Tissue by Polyman 19


2.2. Polyman 19 Toxicity towards Cervical Explants

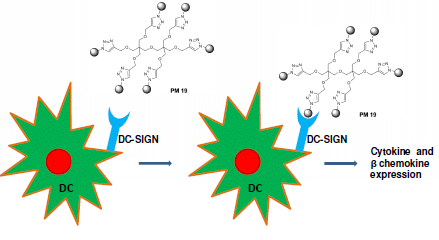
2.3. Modulation of Innate Immune Responses by Polyman 19



3. Experimental
3.1. Human Cervical Explants Infection
3.2. Tetrazolium Salt 1-(4,5-Dimethylthiazol-2-yl)-3,5-diphenyl Formazan (MTT) Assay
3.3. Human Monocyte Isolation
3.4. Differentiation of DCs from Monocytes
3.5. Flow Cytometry
3.6. RNA Extraction and Retrotranscription
3.7. Real Time PCR
4. Conclusions
Acknowledgments
Authors’ Contribution
Conflicts of Interest
References and Notes
- Girard, M.P.; Osmanov, S.; Assossou, O.M.; Kieny, M.P. Human immunodeficiency virus (HIV) immunopathogenesis and vaccine development: A review. Vaccine 2011, 29, 6191–6218. [Google Scholar] [CrossRef]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef]
- Hammer, S.M.; Sobieszczyk, M.E.; Janes, H.; Karuna, S.T.; Mulligan, M.J.; Grove, D.; Koblin, B.A.; Buchbinder, S.P.; Keefer, M.C.; Tomaras, G.D.; et al. Efficacy Trial of a DNA/rAd5 HIV-1 Preventive Vaccine. N. Engl. J. Med. 2013, 369, 2083–2092. [Google Scholar] [CrossRef]
- MacLeod, M.K.; McKee, A.S.; David, A.; Wang, J.; Mason, R.; Kappler, J.W.; Marrack, P. Vaccine adjuvants aluminum and monophosphoryl lipid A provide distinct signals to generate protective cytotoxic memory CD8 T cells. Proc. Natl. Acad. Sci. USA 2011, 108, 7914–7919. [Google Scholar]
- De Gregorio, E.; Caproni, E.; Ulmer, J.B. Vaccine adjuvants: Mode of action. Front. Immunol. 2013, 4, 1–5. [Google Scholar]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Casella, C.R.; Mitchell, T.C. Putting endotoxin to work for us: Monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell. Mol. Life Sci. 2008, 65, 3231–3240. [Google Scholar] [CrossRef]
- Akira, S. Innate immunity and adjuvants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2748–2755. [Google Scholar] [CrossRef]
- Lang, R.; Schoenen, H.; Desel, C. Targeting Syk-Card9-activating C-type lectin receptors by vaccine adjuvants: Findings, implications and open questions. Immunobiology 2011, 216, 1184–1191. [Google Scholar] [CrossRef]
- Van Kooyk, Y.; Unger, W.W.; Fehres, C.M.; Kalay, H.; García-Vallejo, J.J. Glycan-based DC-SIGN targeting vaccines to enhance antigen cross-presentation. Mol. Immunol. 2013, 55, 143–145. [Google Scholar] [CrossRef]
- Van Kooyk, Y.; Geijtenbeek, T.B. DC-SIGN: Escape mechanism for pathogens. Nat. Rev. Immunol. 2003, 3, 697–709. [Google Scholar] [CrossRef]
- Svajger, U.; Anderluh, M.; Jeras, M.; Obermajer, N. C-type lectin DC-SIGN: An adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity. Cell. Signal. 2010, 22, 1397–1405. [Google Scholar] [CrossRef]
- Sattin, S.; Daghetti, A.; Thépaut, M.; Berzi, A.; Sánchez-Navarro, M.; Tabarani, G.; Rojo, J.; Fieschi, F.; Clerici, M.; Bernardi, A. Inhibition of DC-SIGN-mediated HIV infection by a linear trimannoside mimic in a tetravalent presentation. ACS Chem. Biol. 2010, 5, 301–312. [Google Scholar] [CrossRef]
- Berzi, A.; Reina, J.J.; Ottria, R.; Sutkeviciute, I.; Antonazzo, P.; Sanchez-Navarro, M.; Chabrol, E.; Biasin, M.; Trabattoni, D.; Cetin, I.; et al. A glycomimetic compound inhibits DC-SIGN-mediated HIV infection in cellular and cervical explant models. AIDS 2012, 26, 127–137. [Google Scholar]
- Varga, N.; Sutkeviciute, I.; Guzzi, C.; McGeagh, J.; Petit-Haertlein, I.; Gugliotta, S.; Weiser, J.; Angulo, J.; Fieschi, F.; Bernardi, A. Selective targeting of DC-SIGN with mannose-based glycomimetics. Synthesis and interaction studies of bis-benzylamide derivatives of a pseudo-mannobioside. Chemistry 2013, 19, 4786–4797. [Google Scholar] [CrossRef]
- Varga, N.; Sutkeviciute, I.; Ribeiro-Viana, R.; Berzi, A.; Ramdasi, R.; Daghetti, A.; Vettoretti, G.; Amara, A.; Clerici, M.; Rojo, J.; et al. Identification of a promising multivalent inhibitor of the DC-SIGN dependent uptake of HIV-1 and Dengue virus. Biomaterials 2014, in press. [Google Scholar]
- Cocchi, F.; DeVico, A.L.; Garzino-Demo, A.; Arya, S.K.; Gallo, R.C.; Lusso, P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995, 270, 1811–1815. [Google Scholar]
- Lietz, R.; Bayer, W.; Ontikatze, T.; Johrden, L.; Tenbusch, M.; Storcksdieck Genannt Bonsmann, M.; Uberla, K.; Dittmer, U.; Wildner, O. Codelivery of the chemokine CCL3 by an adenovirus-based vaccine improves protection from retrovirus infection. J. Virol. 2012, 86, 1706–1716. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 63–89. [Google Scholar]
- Maue, A.C.; Eaton, S.M.; Lanthier, P.A.; Sweet, K.B.; Blumerman, S.L.; Haynes, L. Proinflammatoryadjuvants enhance the cognate helper activity of aged CD4 T cells. J. Immunol. 2009, 182, 6129–6135. [Google Scholar]
- Ben-Sasson, S.Z.; Hu-Li, J.; Quiel, J.; Cauchetaux, S.; Ratner, M.; Shapira, I.; Dinarello, C.A.; Paul, W.E. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 17119–17124. [Google Scholar]
- Ahlers, J.D.; Dunlop, N.; Alling, D.W.; Nara, P.L.; Berzofsky, J.A. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs: Granulocyte-macrophage colony-stimulating factor and TNF-alpha synergize with IL-12 to enhance induction of cytotoxic T lymphocytes. J. Immunol. 1997, 158, 3947–3958. [Google Scholar]
- Su, B.; Wang, J.; Wang, X.; Jin, H.; Zhao, G.; Ding, Z.; Kang, Y.; Wang, B. The effects of IL-6 and TNF-alpha as molecular adjuvants on immune responses to FMDV and maturation of dendritic cells by DNA vaccination. Vaccine 2008, 26, 5111–5122. [Google Scholar] [CrossRef]
- Devito, C.; Broliden, K.; Kaul, R.; Svensson, L.; Johansen, K.; Kiama, P.; Kimani, J.; Lopalco, L.; Piconi, S.; Bwayo, J.J.; et al. Mucosal and plasma IgA from HIV-1-exposed uninfected individuals inhibit HIV-1 transcytosis across human epithelial cells. J. Immunol. 2000, 165, 5170–5176. [Google Scholar]
- Miyazawa, M.; Lopalco, L.; Mazzotta, F.; Lo Caputo, S.; Veas, F.; Clerici, M. The “immunologic advantage” of HIV-exposed seronegative individuals. AIDS 2009, 23, 161–175. [Google Scholar]
- Brooks, D.G.; Lee, A.M.; Elsaesser, H.; McGavern, D.B.; Oldstone, M.B. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J. Exp. Med. 2008, 205, 533–541. [Google Scholar] [CrossRef]
- Landay, A.L.; Clerici, M.; Hashemi, F.; Kessler, H.; Berzofsky, J.A.; Shearer, G.M. In vitro restoration of T cell immune function in human immunodeficiency virus-positive persons: Effects of interleukin (IL)-12 and anti-IL-10. J. Infect. Dis. 1996, 173, 1085–1091. [Google Scholar] [CrossRef]
- Boasso, A.; Shearer, G.M.; Clerici, M. The hunt for an HIV vaccine: Time to rethink recent failures. Lancet 2008, 371, 1897–1898. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Berzi, A.; Varga, N.; Sattin, S.; Antonazzo, P.; Biasin, M.; Cetin, I.; Trabattoni, D.; Bernardi, A.; Clerici, M. Pseudo-Mannosylated DC-SIGN Ligands as Potential Adjuvants for HIV Vaccines. Viruses 2014, 6, 391-403. https://doi.org/10.3390/v6020391
Berzi A, Varga N, Sattin S, Antonazzo P, Biasin M, Cetin I, Trabattoni D, Bernardi A, Clerici M. Pseudo-Mannosylated DC-SIGN Ligands as Potential Adjuvants for HIV Vaccines. Viruses. 2014; 6(2):391-403. https://doi.org/10.3390/v6020391
Chicago/Turabian StyleBerzi, Angela, Norbert Varga, Sara Sattin, Patrizio Antonazzo, Mara Biasin, Irene Cetin, Daria Trabattoni, Anna Bernardi, and Mario Clerici. 2014. "Pseudo-Mannosylated DC-SIGN Ligands as Potential Adjuvants for HIV Vaccines" Viruses 6, no. 2: 391-403. https://doi.org/10.3390/v6020391
APA StyleBerzi, A., Varga, N., Sattin, S., Antonazzo, P., Biasin, M., Cetin, I., Trabattoni, D., Bernardi, A., & Clerici, M. (2014). Pseudo-Mannosylated DC-SIGN Ligands as Potential Adjuvants for HIV Vaccines. Viruses, 6(2), 391-403. https://doi.org/10.3390/v6020391