Hepatitis A Immunity in the District of Aveiro (Portugal): An Eleven-Year Surveillance Study (2002–2012)
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Sample


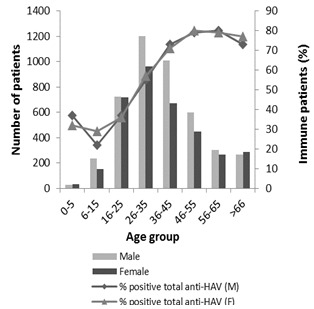
2.2. Characterization of HAV Immune Patients


2.3. Characterization of Infected Patients


3. Discussion
4. Materials and Methods
4.1. Samples
4.2. Sampling
4.3. Antibodies Detection
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Bower, W.A.; Nainan, O.V.; Han, X.; Margolis, H.S. Duration of viremia in hepatitis A virus infection. J. Infect. Dis. 2000, 182, 12–17. [Google Scholar] [CrossRef]
- Wasley, A.; Samandari, T.; Bell, B.P. Incidence of hepatitis A in the United States in the era of vaccination. J. Am. Med. Assoc. 2005, 294, 194–201. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 1999, 48, 1–37. [Google Scholar]
- Bell, B.P.; Shapiro, C.N.; Alter, M.J.; Moyer, L.; Judson, F.N.; Mottram, K.; Fleenor, M.; Ryder, P.L.; Margolis, H.S. The diverse patterns of hepatitis A epidemiology in the United States-implications for vaccination strategies. J. Infect. Dis. 1998, 178, 1579–1584. [Google Scholar] [CrossRef]
- Cuthbert, J.A. Hepatitis A: Old and new hepatitis A. Clin. Microbiol. Rev. 2001, 14, 38–58. [Google Scholar] [CrossRef]
- Ciocca, M. Clinical course and consequences of hepatitis A infection. Vaccine 2000, 18, S71–S74. [Google Scholar] [CrossRef]
- Rosenthal, P. Cost-effectiveness of hepatitis A vaccination in children, adolescents, and adults. Hepatology 2003, 37, 44–51. [Google Scholar] [CrossRef]
- Luyten, J.; Beutels, P. Costing infectious disease outbreaks for economic evaluation. Pharmacoeconomics 2009, 27, 379–389. [Google Scholar] [CrossRef]
- FitzSimons, D.; Hendrickx, G.; Vorsters, A.; van Damme, P. Hepatitis A and E: Update on prevention and epidemiology. Vaccine 2010, 28, 583–588. [Google Scholar] [CrossRef]
- World Health Organization. The Global Prevalence of Hepatitis A Virus Infection and Susceptibility: A Systematic Review; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Marinho, R.; Ramalho, F.; Moura, M.C.; Valente, A. Hepatite A.: Alteração do padrão epidemiológico? Revist. Portug. Clín. Geral 2000, 16, 103–111. [Google Scholar]
- Lecour, H.; Ribeiro, T.; Amaral, I.; Rodrigues, M. Prevalence of viral hepatitis markers in the population of Portugal. Bull. World Health Org. 1984, 62, 743–747. [Google Scholar]
- Raczniak, G.; Thomas, T.K.; Bulkow, L.R.; Negus, S.E.; Zanis, C.L.; Bruce, M.G.; Spradling, P.R.; Teshale, E.H.; McMahon, B.J. Duration of protection against hepatitis A for the current two-dose vaccine compared to a three-dose vaccine schedule in children. Vaccine 2013, 31, 2152–2155. [Google Scholar]
- Landry, P.; Tremblay, S.; Darioli, R.; Genton, B. Inactivated hepatitis A vaccine booster given >/=24 months after the primary dose. Vaccine 2000, 19, 399–402. [Google Scholar] [CrossRef]
- Kallinowski, B.; Knöll, A.; Lindner, E.; Sänger, R.; Stremmel, W.; Vollmar, J.; Zieger, B.; Jilg, W. Can monovalent hepatitis A and B vaccines be replaced by a combined hepatitis A/B vaccine during the primary immunization course? Vaccine 2000, 19, 16–22. [Google Scholar] [CrossRef]
- Bauch, C.T.; Anonychuk, M.; Pham, B.Z.; Gilca, V.; Duval, B.; Krahn, M.D. Cost-utility of universal hepatitis A vaccination in Canada. Vaccine 2007, 25, 8536–8548. [Google Scholar] [CrossRef]
- Jacobs, R.J.; Margolis, H.S. The cost-effectiveness of adolescent hepatitis A vaccination in states with the highest disease rates. Arch. Paediatr. Adolesc. Med. 2000, 154, 763–770. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Burgess, M.A.; Hull, B. Hepatitis A vaccination options for Australia. J. Paediatr. Child Health 2003, 39, 83–87. [Google Scholar] [CrossRef]
- Navas, E.; Salleras, L.; Gisbert, R.; Dominguez, A.; Bruguera, M.; Rodríguez, G.; Galí, N.; Prat, A. Efficiency of the incorporation of the hepatitis A vaccine as a combined A + B vaccine to the hepatitis B vaccination programme of preadolescents in schools. Vaccine 2005, 23, 2185–2189. [Google Scholar] [CrossRef]
- Cavaco, A.; Manso, A.; Rodrigues, F.; Marques, J.G.; Marques, L. Recomendações sobre vacinas. Soc. Portug. Pediatr. 2010, 1, 10–13. [Google Scholar]
- Direção Geral da Saúde. Programa Nacional de Vacinação 2012. Norma da Direção Geral da Saúde: Lisboa, Portugal, 2012. [Google Scholar]
- Rodrigues, L. Vírus da hepatite A. Avaliação do Programa Nacional de Vacinação e Melhoria do seu custo-efectividade. 2° Inquérito Serológico Nacional-Portugal Continental 2001–2002. Direção Geral Saúde: Lisboa, Portugal, 2004; pp. 113–122. [Google Scholar]
- CDC. Prevention of hepatitis A through active or passive immunization. Morbid. Mortal. Weekly Rep. 1999, 48, 1–37. [Google Scholar]
- Operator’s Guide. ADVIA Centaur® XP Immunoassay System. 2006. Available online: http://healthcare.siemens.com/immunoassay/systems/advia-centaur-xp/ (accessed on 20 June 2013).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pereira, S.; Linhares, I.; Neves, A.F.; Almeida, A. Hepatitis A Immunity in the District of Aveiro (Portugal): An Eleven-Year Surveillance Study (2002–2012). Viruses 2014, 6, 1336-1345. https://doi.org/10.3390/v6031336
Pereira S, Linhares I, Neves AF, Almeida A. Hepatitis A Immunity in the District of Aveiro (Portugal): An Eleven-Year Surveillance Study (2002–2012). Viruses. 2014; 6(3):1336-1345. https://doi.org/10.3390/v6031336
Chicago/Turabian StylePereira, Sara, Inês Linhares, António Ferreira Neves, and Adelaide Almeida. 2014. "Hepatitis A Immunity in the District of Aveiro (Portugal): An Eleven-Year Surveillance Study (2002–2012)" Viruses 6, no. 3: 1336-1345. https://doi.org/10.3390/v6031336
APA StylePereira, S., Linhares, I., Neves, A. F., & Almeida, A. (2014). Hepatitis A Immunity in the District of Aveiro (Portugal): An Eleven-Year Surveillance Study (2002–2012). Viruses, 6(3), 1336-1345. https://doi.org/10.3390/v6031336