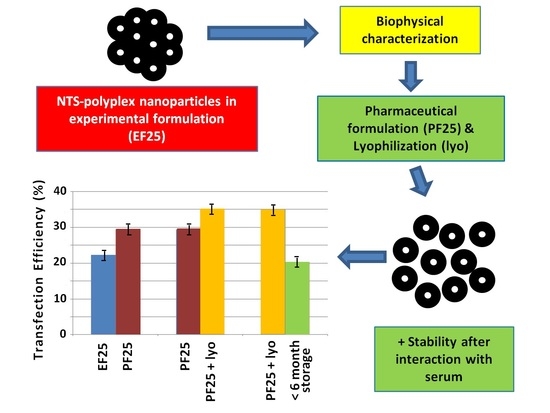
Development of a Parenteral Formulation of NTS-Polyplex Nanoparticles for Clinical Purpose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of NTS-Carrier and Formation of NTS-Polyplex NPs
2.3. Biological Functionality In Vitro
2.4. Biological Functionality In Vivo
Animals
2.5. Transmission Electron Microscopy
2.6. Dynamic Light Scattering
2.7. Electrophoretic Mobility
2.8. Circular Dichroism (CD)
2.9. Lyophilization Protocol
2.10. Size Exclusion Chromatography Using a Radioactive Tag
3. Results
3.1. Formulation of NTS-Polyplex NPs
3.2. Biological Functionality In Vitro and In Vivo
3.3. Lyophilization of NTS-Polyplex NPs
3.4. Effect of Lyophilization on Biological Activity of NTS-Polyplex NPs In Vitro and In Vivo
3.5. Physical Structure of NTS-Polyplex NPs
3.6. Size and Z-Potential of NTS-Polyplex NPs
3.7. Secondary Structure of NTS-Polyplex NPs
3.8. Stability of Reconstituted NTS-Polyplex NPs after Interaction with Serum
3.9. Stability Tests of Lyophilisates of NTS-Polyplex NPs in PF25
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martinez-Fong, D.; Navarro-Quiroga, I.; Ochoa, I.; Alvarez-Maya, I.; Meraz, M.A.; Luna, J.; Arias-Montano, J.A. Neurotensin-SPDP-poly-l-lysine conjugate: A nonviral vector for targeted gene delivery to neural cells. Mol. Brain Res. 2009, 69, 249–262. [Google Scholar] [CrossRef]
- Martinez-Fong, D.; Navarro-Quiroga, I. Synthesis of a non-viral vector for gene transfer via the high-affinity neurotensin receptor. Brain Res. Protoc. 2000, 6, 13–24. [Google Scholar] [CrossRef]
- Hernandez-Baltazar, D.; Martinez-Fong, D.; Trudeau, L.E. Optimizing NTS-polyplex as a tool for gene transfer to cultured dopamine neurons. PLoS ONE 2012, 7, e51341. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Fong, D.; Bannon, M.J.; Trudeau, L.E.; Gonzalez-Barrios, J.A.; Arango-Rodriguez, M.L.; Hernandez-Chan, N.G.; Reyes-Corona, D.; Armendariz-Borunda, I.; Navarro-Quiroga, I. NTS-Polyplex: A potential nanocarrier for neurotrophic therapy of Parkinson’s disease. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1052–1069. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Quiroga, I.; Gonzalez-Barrios, J.A.; Barron-Moreno, F.; Gonzalez-Bernal, V.; Martinez-Arguelles, D.B.; Martinez-Fong, D. Improved neurotensin-vector-mediated gene transfer by the coupling of hemagglutinin HA2 fusogenic peptide and Vp1 SV40 nuclear localization signal. Mol. Brain Res. 2002, 105, 86–97. [Google Scholar] [CrossRef]
- Gonzalez-Barrios, J.; Bannon, M.; Anaya-Martínez, V.; Flores, G. Neurotensin Polyplex as an Efficient Carrier for Delivering the Human GDNF Gene into Nigral Dopamine Neurons of Hemiparkinsonian Rats. Mol. Ther. 2006, 6, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Chan, N.; Bannon, M.; Orozco-Barrios, C.; Escobedo, L.; Zamudio, S.; Cruz, F.D.I.; Gongora-Alfaro, J.; Armendariz-Borunda, J.; Reyes-Corona, D.; Espadas-Alvarez, A.; et al. Neurotensin-polyplex-mediated brain-derived neurotrophic factor gene delivery into nigral dopamine neurons prevents nigrostriatal degeneration in a rat model of early Parkinson’s disease. J. Biomed. Sci. 2015, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Razgado-Hernandez, L.; Espadas-Alvarez, A.; Reyna-Velazquez, P.; Sierra-Sanchez, A.; Anaya-Martinez, V.; Jimenez-Estrada, I.; Bannon, M.; Martinez-Fong, D.; Aceves-Ruiz, J. The transfection of BDNF to dopamine neurons potentiates the effect of dopamine D3 receptor agonist recovering the striatal innervation, dendritic spines and motor behavior in an aged rat model of Parkinson’s disease. PLoS ONE 2015, 10, e0117391. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Rodriguez, R.A.; Arango-Rodriguez, M.L.; Escobedo, L.; Hernandez-Baltazar, D.; Gompel, A.; Forgez, P.; Martinez-Fong, D. Suicide HSVtk gene delivery by neurotensin-polyplex nanoparticles via the bloodstream and GCV Treatment specifically inhibit the growth of human MDA-MB-231 triple negative breast cancer tumors xenografted in athymic mice. PLoS ONE 2014, 9, e97151. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Zapata, H.; Rembao-Bojorquez, J.; Arango-Rodríguez, M.; Dupoy, S. NT-polyplex: A new tool for therapeutic gene delivery to neuroblastoma tumors. Cancer Gene Ther. 2009, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Arango-Rodriguez, M.L.; Navarro-Quiroga, I.; Gonzalez-Barrios, J.A.; Martinez-Arguelles, D.B.; Bannon, M.; Kouri, J.; Forgez, P.; Rostene, W.; Garcia-Villegas, R.; Jimenez, I.; et al. Biophysical characteristics of neurotensin polyplex for in vitro and in vivo gene transfection. Biochem. Biophys. Acta 2006, 1760, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.; Rembao, J.; Hernandez-Baltazar, D.; Castillo-Rodriguez, R.; Tellez-Lopez, V.; Flores-Martinez, Y.; Orozco-Barrios, C.; Rubio, H.; Sanchez-Garcia, A.; Ayala-Davila, J.; et al. Safety of the intravenous administration of neurotensin-polyplex nanoparticles in BALB/c mice. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Espadas-Alvarez, A.; Bannon, M.; Orozco-Barrios, C.; Escobedo-Sanchez, L.; Ayala-Davila, J.; Reyes-Corona, D.; Soto-Rodriguez, G.; Escamilla-Rivera, V.; Vizcaya-Ruiz, A.D.; Gutierrez-Castillo, M.; et al. Regulation of human GDNF gene expression in nigral dopaminergic neurons using a new doxycycline-regulated NTS-polyplex nanoparticle system. Nanomed. Nanotechnol. Biol. Med. 2017, 17, 30025. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.; Dubin, D. The Role of Polyamines in the Neutralization of Bacteriophage Deoxiribonucleic Acid. J. Biol. Chem. 1960, 235, 769–775. [Google Scholar] [PubMed]
- De Ilarduya, C.T.; Sun, Y.; Duzgunes, N. Gene delivery by lipoplexes and polyplexes. Eur. J. Pharm. Sci. 2010, 40, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Anchordoquy, T.J.; Koe, G. Physical Stability of Nonviral Plasmid-Based Therapeutics. J. Pharm. Sci. 1999, 89, 289–296. [Google Scholar] [CrossRef]
- Brus, C.; Aigner, K.E.; Czubayko, F.; Kissel, T. Stabilization of oligonucleotide-polyethylenimine complexes by freeze-drying: Physicichemical and biological characterization. J. Controll. Release 2004, 95, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.C.; Pikal, M.J.; Friess, W. Development of a lyophililed plasmid/LPEI polyplex formulation with long-term stability—A step closer from promising technology to application. J. Controll. Release 2011, 151, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Hud, N.; Vilfan, I. Toroidal DNA condensates: Unraveling the fine structure and the role of nucleation in determining size. Annu. Rev. Biophys. Biomol. Struct. 2005, 34, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Goula, D.; Remy, J.S.; Erbacher, P.; Wazowicz, M.; Levi, G.; Abdallah, B.; Demeneix, B.A. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene Ther. 1998, 5, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Hartikka, J.; Bozoukova, V.; Jones, D.; Mahajan, R.; Wloch, M.K.; Sawdey, M.; Buchner, C.; Sukhu, L.; Barnhart, K.M.; Abai, A.M.; et al. Sodium phosphate enhances plasmid DNA expression in vivo. Gene Ther. 2000, 7, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Talsma, H.; Cherng, J.; Lehrmann, H.; Kursa, M.; Ogris, M.; Hennink, W.E.; Cotton, M.; Wagner, E. Stabilization of gene delivery systems by freeze-drying. Int. J. Pharm. 1997, 157, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.C.; Pikal, M.J.; Friess, W. Investigations on polyplex stability during the freezing step of lyophilization using controlled ice nucleation—The importance of residence time in the low-viscosity fluid state. J. Pharm. Sci. 2013, 102, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.; Anchordoquy, T. Mechasnisms of protection of cationic lipid-DNA complexes during lyophilization. J. Pharm. Sci. 2000, 89, 682–691. [Google Scholar] [CrossRef]
- Anchordoquy, T.; Allison, D.; Molina, M.; Girouard, L.D.; Carson, T.K. Physical stabilization of DNA-based therapeutics. Drug Discov. Today 2001, 6, 463–470. [Google Scholar] [CrossRef]
- Anchordoquy, T.; Armstrong, T.; Molina, M. Low Molecular weight dextrans stabilize nonviral vectors during lyophilization at low osmolality: Concentrating suspensions by rehydration to reduce volumes. J. Pharm. Sci. 2005, 94, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Cusack, B.; Stanton, T.; Richelson, E. Developmental regulation of neurotensin receptor expression and function in murine neuroblastoma clone N1E-115. Eur. J. Pharmacol. 1991, 206, 339–342. [Google Scholar] [CrossRef]
- Toy-Miou-Leong, M.; Cortes, M.; Baudet, A.; Rostene, W.; Forgez, P. Receptor trafficking via the perinuclear recycling compartment accompanied by cell division is necessary for permanent neurotensin cell sensitization and leads to chronic mitogen-activated protein kinase activation. J. Biol. Chem. 2004, 279, 12636–12646. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Inactive Ingredient Search for Approved Drug Products. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/ (accessed on 7 November 2017).
- Allison, S.; Marion, D.; Molina, M.; Anchordoquy, T. Stabilization of lipid/DNA complexes during freezing step ot the lyophilization process: The particle isolation hypothesis. Biochem. Biophys. Acta 2000, 1468, 127–138. [Google Scholar] [CrossRef]
- Anchordoquy, T.; Carpenter, J.; Kroll, D. Maintenance of transfection rates and physical characterization of lipid/DNA complexes after freeze-drying and rehydration. Arch. Biochem. Byophys. 1997, 348, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Maya, I.; Navarro-Quiroga, M.; Meraz-Ríos, J.; Aceves, J.; Martínez-Fong, D. In vivo gene transfer to dopamine neurons of rat substantia nigra via the high-affinity neurotensin receptor. Mol. Med. 2001, 7, 186–192. [Google Scholar]
- Chattoraj, D.; Gosule, L.; Schellman, J. DNA condensation with polyamines. J. Mol. Biol. 1978, 121, 327–337. [Google Scholar] [CrossRef]
- Danielsen, S.; Maurstad, G.; Stokke, B. DNA-polycation complexation and polyplex stability in the presence of competing polyanions. Biopolymers 2005, 77, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Golan, R.; Pietrasanta, L.; Hsieh, W.; Hansma, H. DNA toroids: Stages in condensation. Biochemistry 1999, 38, 14069–14076. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U. Characterization of DNA condensates induced by poly(ethyene oxide) and polylysine. Proc. Natl. Acad. Sci. USA 1975, 72, 4288–4292. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Molas, M.; Grossman, G.; Pasumarthy, M.; Perales, J.; Cooper, M.; Hanson, R. Biological properties of poly-L-lysine-DNA complexes generated by cooperative binding of the polycation. J. Biol. Chem. 2001, 276, 34379–34387. [Google Scholar] [CrossRef] [PubMed]
- Maestre, M.; Wang, J. Circular dichroism of superhelical DNA. Biopolymers 1971, 10, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Ausar, S.; Joshi, S.; Middaugh, C. Spectroscopic methods for the physical characterization and formulation of nonviral gene delivery systems. Methods Mol. Biol. 2008, 434, 55–80. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.S.; Aron, Y.; Thevenot, G.; Francois, D.; Monsigny, M.; Fajac, I. Potocytosis and cellular exit of complexes as cellular pathways for gene delivery by polycations. J. Gene Med. 2005, 7, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Phillip, A.; Wagner, E. Receptor-Targeted Polyplexes for DNA and siRNA Delivery. In Gene and Cell Therapy; Templeton, N., Ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 341–355. [Google Scholar] [CrossRef]
- Conwell, C.; Vilfan, I.; Hud, N. Controlling Size of nanoscale toroidal DNA condensates with static curvature and ionic strength. Proc. Natl. Acad. Sci. USA 2003, 100, 9296–9301. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.; Anchordoquy, T. Lyophilization of nonviral gene delivery systems. Methods Mol. Med. 2001, 65, 225–252. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.; Allison, S.; Anchordoquy, T. Maintenance of nonviral vector particle size during the freezong step of the lyophilization process is insufficient for preservation of activity: Insight from other structural indicators. J. Pharm. Sci. 2001, 90, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.; Summers, H.; Godin, B. Interactions of Cationic Polymers with Cells. In Cationic Polymers in Regenerative Medicine; Samal, S., Bubruel, P., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2015; Chapter 18; pp. 472–502. [Google Scholar]
- Bertin, A. Polyelectrolite Complexes of DNA and Polycations as Gene Delivery Vectors. In Polyelectrolite Complexes in the Dispersed and Solid State II; Muller, M., Ed.; Springer: Brelin, Germany, 2014; Volume 256, pp. 103–196. [Google Scholar]
- Frere, A.; Evrard, B.; Mottet, D.; Piel, G. Polymeric Nanoparticles as siRNA Drug Delivery System for Cancer Therapy: The Long Road to Therapeutic Efficiency. In Nanoarchitectonics for Smart Delivery and Drug Targeting; Holban, A., Grumezescu, A., Eds.; Elsevier: Oxford, UK, 2016; Chapter 18; pp. 503–532. [Google Scholar]
- Armstrong, T.; Anchordoquy, T. Immobilization of nonviral vectors during the freezing step of lyophilization. J. Pharm. Sci. 2004, 93, 2698–2709. [Google Scholar] [CrossRef] [PubMed]
- Davis, M. Non-viral gene delivery systems. Curr. Opin. Biotechnol. 2002, 13, 128–131. [Google Scholar] [CrossRef]
- Zuckerman, J.; Choi, C.; Han, H.; Davis, M. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 3137–3142. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S. Molecular Mobility of Freeze-Dried Formulations Determined by NMR Relaxation and Its Effect on Storage Stability. In Freeze-Drying/Lyophilization of Pharmaceutical & Biological Products, 2nd ed.; Rey, L., May, J.C., Eds.; CRC Press: Boca Ratón, FL, USA, 2004; pp. 203–205. [Google Scholar]








| Component | Function | Examples | Selected Ingredient(s) | Reference |
|---|---|---|---|---|
| Stabilizer | Control of NPs size | Salts | NaCl, KCl, CaCl2, MgCl2, NaH2PO4 | [19] |
| Buffer | Preserve pH balance | Phosphates, citrates, amino acids | NaH2PO4 | [21] |
| Tonicity modifiers | Provide Isotonicity | Salts, saccharides, polyols, polymers | NaCl, glucose | [26] |
| Cryo-and lyoprotectant/Bulking agent | Avoid fracturing | Saccharides, polyols, polymers, amino acids | Glucose | [17,18,22,23,24,25,26] |
| Vehicle | Glucose-DNA Ratio | Osmolarity (mOsm/L) |
|---|---|---|
| PSS | Not applicable | 289.2 |
| EF25 | 241.8 | 283 |
| PF25 | 280.6 | |
| EF280 | 2710.6 | 480.6 |
| PF280 | 501.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranda-Barradas, M.E.; Márquez, M.; Quintanar, L.; Santoyo-Salazar, J.; Espadas-Álvarez, A.J.; Martínez-Fong, D.; García-García, E. Development of a Parenteral Formulation of NTS-Polyplex Nanoparticles for Clinical Purpose. Pharmaceutics 2018, 10, 5. https://doi.org/10.3390/pharmaceutics10010005
Aranda-Barradas ME, Márquez M, Quintanar L, Santoyo-Salazar J, Espadas-Álvarez AJ, Martínez-Fong D, García-García E. Development of a Parenteral Formulation of NTS-Polyplex Nanoparticles for Clinical Purpose. Pharmaceutics. 2018; 10(1):5. https://doi.org/10.3390/pharmaceutics10010005
Chicago/Turabian StyleAranda-Barradas, María E., Maripaz Márquez, Liliana Quintanar, Jaime Santoyo-Salazar, Armando J. Espadas-Álvarez, Daniel Martínez-Fong, and Elizabeth García-García. 2018. "Development of a Parenteral Formulation of NTS-Polyplex Nanoparticles for Clinical Purpose" Pharmaceutics 10, no. 1: 5. https://doi.org/10.3390/pharmaceutics10010005
APA StyleAranda-Barradas, M. E., Márquez, M., Quintanar, L., Santoyo-Salazar, J., Espadas-Álvarez, A. J., Martínez-Fong, D., & García-García, E. (2018). Development of a Parenteral Formulation of NTS-Polyplex Nanoparticles for Clinical Purpose. Pharmaceutics, 10(1), 5. https://doi.org/10.3390/pharmaceutics10010005