Influence of Solvent Composition on the Performance of Spray-Dried Co-Amorphous Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Methods
2.2.1. Ball-Milling
2.2.2. Spray-Drying
2.3. Analytical Methods
2.3.1. X-ray Powder Diffraction (XRPD)
2.3.2. Modulated Differential Scanning Calorimetry (mDSC)
2.3.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.4. Powder Dissolution
2.4. Storage
3. Results and Discussion
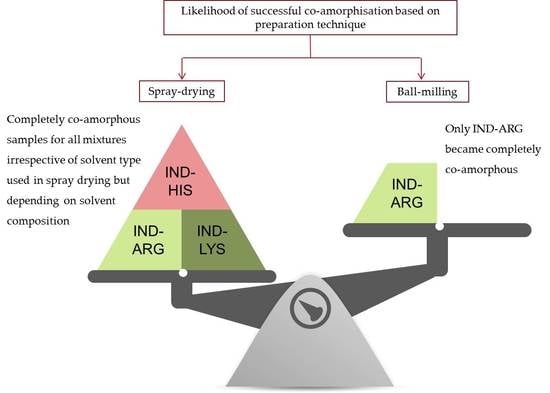
3.1. Preparation of Co-Amorphous Formulations
3.2. Thermal Analysis of Co-Amorphous Systems
3.3. Investigation of Molecular Interactions by FTIR Spectroscopy
3.4. Dissolution Rate
3.5. Physical Stability During Storage
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Colombo, P.; Sonvico, F.; Colombo, G.; Bettini, R. Novel Platforms for Oral Drug Delivery. Pharm. Res. 2009, 26, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Perioli, L.; D’Alba, G.; Pagano, C. New oral solid dosage form for furosemide oral administration. Eur. J. Pharm. Biopharm. 2012, 80, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Naseem, A.; Olliff, C.J.; Martini, L.G.; Lloyd, A.W. Effects of plasma irradiation on the wettability and dissolution of compacts of griseofulvin. Int. J. Pharm. 2004, 269, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N. Strategies to Address Low Drug Solubility in Discovery and Development. Pharmacogn. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef]
- Hancock, B.C.; Zografi, G. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.H.; Rades, T.; Müllertz, A. Stabilisation of amorphous furosemide increases the oral drug bioavailability in rats. Int. J. Pharm. 2015, 490, 334–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Löbmann, K.; Grohganz, H.; Laitinen, R.; Strachan, C.; Rades, T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs—Part 1: Preparation, stability and dissolution enhancement. Eur. J. Pharm. Biopharm. 2013, 85, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Löbmann, K.; Laitinen, R.; Grohganz, H.; Strachan, C.; Rades, T.; Gordon, K.C. A theoretical and spectroscopic study of co-amorphous naproxen and indomethacin. Int. J. Pharm. 2013, 453, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.T.; Blaabjerg, L.I.; Lenz, E.; Bohr, A.; Grohganz, H.; Kleinebudde, P.; Rades, T.; Löbmann, K. Preparation and characterization of spray-dried co-amorphous drug-amino acid salts. J. Pharm. Pharmacol. 2016, 68, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.T.; Löbmann, K.; Rades, T.; Grohganz, H. Improving co-amorphous drug formulations by the addition of the highly water soluble amino acid, Proline. Pharmaceutics 2014, 6, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Löbmann, K.; Laitinen, R.; Strachan, C.; Rades, T.; Grohganz, H. Amino acids as co-amorphous stabilizers for poorly water-soluble drugs—Part 2: Molecular interactions. Eur. J. Pharm. Biopharm. 2013, 85, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Chieng, N.; Aaltonen, J.; Saville, D.; Rades, T. Physical characterization and stability of amorphous indomethacin and ranitidine hydrochloride binary systems prepared by mechanical activation. Eur. J. Pharm. Biopharm. 2009, 71, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.W.; Löbmann, K.; Grohganz, H.; Rades, T.; Chieng, N. Investigation of physical properties and stability of indomethacin-cimetidine and naproxen-cimetidine co-amorphous systems prepared by quench cooling, coprecipitation and ball milling. J. Pharm. Pharmacol. 2016, 68, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, A.; Ambike, A.A.; Jadhav, B.K.; Mahadik, K.R. Characterization of curcumin–PVP solid dispersion obtained by spray drying. Int. J. Pharm. 2004, 271, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, H.J.; Cho, W.; Cha, K.H.; Kang, Y.S.; Hwang, S.J. Preparation and pharmaceutical characterization of amorphous cefdinir using spray-drying and SAS-process. Int. J. Pharm. 2010, 396, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.B.; Patel, J.K.; Chakraborty, S.; Shukla, D. Revealing facts behind spray dried solid dispersion technology used for solubility enhancement. Saudi Pharm. J. 2015, 23, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Worku, Z.A.; Aarts, J.; Singh, A.; Van den Mooter, G. Drug–Polymer Miscibility across a Spray Dryer: A Case Study of Naproxen and Miconazole Solid Dispersions. Mol. Pharm. 2014, 11, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Craye, G.; Löbmann, K.; Grohganz, H.; Rades, T.; Laitinen, R. Characterization of amorphous and co-amorphous simvastatin formulations prepared by spray drying. Molecules 2015, 20, 21532–21548. [Google Scholar] [CrossRef] [PubMed]
- Sou, T.; Kaminskas, L.M.; Nguyen, T.-H.; Carlberg, R.; McIntosh, M.P.; Morton, D.A.V. The effect of amino acid excipients on morphology and solid-state properties of multi-component spray-dried formulations for pulmonary delivery of biomacromolecules. Eur. J. Pharm. Biopharm. 2013, 83, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Boraey, M.A.; Hoe, S.; Sharif, H.; Miller, D.P.; Lechuga-Ballesteros, D.; Vehring, R. Improvement of the dispersibility of spray-dried budesonide powders using leucine in an ethanol–water cosolvent system. Powder Technol. 2013, 236, 171–178. [Google Scholar] [CrossRef]
- Klein, S.; Shah, V.P. A standardized mini paddle apparatus as an alternative to the standard paddle. AAPS PharmSciTech 2008, 9, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.; Taylor, J.S. Ideal Copolymers and the Second-order Transitions of Synthetic Rubbers. I. Non-Crystalline Copolymers. J. Appl. Chem. 1952, 2, 493–500. [Google Scholar] [CrossRef]
- Patterson, J.E.; James, M.B.; Forster, A.H.; Lancaster, R.W.; Butler, J.M.; Rades, T. Preparation of glass solutions of three poorly water soluble drugs by spray drying, melt extrusion and ball milling. Int. J. Pharm. 2007, 336, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Izutsu, K.I.; Fujimaki, Y.; Kuwabara, A.; Aoyagi, N. Effect of counterions on the physical properties of L-arginine in frozen solutions and freeze-dried solids. Int. J. Pharm. 2005, 301, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Mattern, M.; Winter, G.; Kohnert, U.; Lee, G. Formulation of proteins in vacuum-dried glasses. II. Process and storage stability in sugar-free amino acid systems. Pharm. Dev. Technol. 1999, 4, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Bahl, D.; Bogner, R.H. Amorphization alone does not account for the enhancement of solubility of drug co-ground with silicate: The case of indomethacin. AAPS PharmSciTech 2008, 9, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.; Zografi, G. A study of amorphous molecular dispersions of indomethacin and its sodium salt. J. Pharm. Sci. 2001, 90, 1991–2004. [Google Scholar] [CrossRef] [PubMed]
- Bhattachar, S.N.; Deschenes, L.A.; Wesley, J.A. Solubility: It’s not just for physical chemists. Drug Discov. Today 2006, 11, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Caron, V.; Tajber, L.; Corrigan, O.I.; Healy, A.M. A Comparison of Spray Drying and Milling in the Production of Amorphous Dispersions of Sulfathiazole/Polyvinylpyrrolidone and Sulfadimidine/Polyvinylpyrrolidone. Mol. Pharm. 2011, 8, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Q.; Wang, J.-R.; Lin, K.-L.; Mei, X. Amino acids as co-amorphous excipients for tackling the poor aqueous solubility of valsartan Amino acids as co-amorphous excipients for tackling the poor aqueous solubility of valsartan. Pharm. Dev. Technol. 2017, 22, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Löbmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef] [PubMed]






| IND–AA Mixtures | Solvent Mixture | ORGANIC Solvent Content %:Water Content % (v/v) | Concentration (mg/mL) in the Spray-Drying Solution | XRPD | Tg by mDSC (°C) | Tg by GT (°C) | FTIR |
|---|---|---|---|---|---|---|---|
| IND–ARG | Acetone + water | 85:15 | 51.4 | A | 115.6 ± 0.6 | 41.3 | SF |
| 70:30 | 38.7 | A | 115.4 ± 1.2 | 41.3 | SF | ||
| 55:45 | 14 | A | 116.4 ± 0.4 | 41.3 | SF | ||
| 45:55 | 9 | A | 116.7 ± 0.7 | 41.3 | SF | ||
| 30:70 | 7 | A | 116.5 ± 0.6 | 41.3 | SF | ||
| 15:85 | 4.8 | A | 115.7 ± 0.5 | 41.3 | SF | ||
| IND–HIS | Acetone + water | 85:15 | 9 | C | - | - | PoNSF |
| 70:30 | 12 | A | 99.8 ± 0.4 | 35.9 | PoNSF | ||
| 55:45 | 14.8 | A | 101.9 ± 0.7 | 35.9 | PoNSF | ||
| 45:55 | 18 | A | 95.1 ± 1.3 | 35.9 | PoNSF | ||
| 30:70 | 21 | A | D: 49.6 ± 1.4, 110.8 ± 0.4 | - | PoNSF | ||
| 15:85 | 25 | C | - | - | PoNSF | ||
| IND–LYS | Acetone + water | 85:15 | 1.92 | C | - | - | SF |
| 70:30 | 12 | A | 98.1 ± 1.3 | 41.8 | SF | ||
| 55:45 | 20 | A | 99.1 ± 1.6 | 41.8 | SF | ||
| 45:55 | 25 | A | 97.5 ± 0.8 | 41.8 | SF | ||
| 30:70 | 29 | A | 96.8 ± 0.6 | 41.8 | SF | ||
| 15:85 | 36 | C | - | 41.8 | SF | ||
| IND–ARG | Ethanol + water | 95:05 | 15 | A | 116.6 ± 1.4 | 41.3 | SF |
| 90:10 | 12 | A | 114.8 ± 1.8 | 41.3 | SF | ||
| 80:20 | 9 | A | 114.2 ± 1.2 | 41.3 | SF | ||
| 60:40 | 7 | A | 118.1 ± 0.7 | 41.3 | SF | ||
| 40:60 | 5 | A | 116.7 ± 1.2 | 41.3 | SF | ||
| 20:80 | 3 | A | 116.1 ± 0.8 | 41.3 | SF | ||
| 10:90 | 2 | A | 115.9 ± 0.4 | 41.3 | SF | ||
| 05:95 | 1.2 | A | 115.4 ± 0.6 | 41.3 | SF | ||
| IND–HIS | Ethanol + water | 95:05 | 13.5 | C | - | - | PoNSF |
| 90:10 | 13 | C | - | - | PoNSF | ||
| 80:20 | 14.5 | A | 96.7 ± 0.4 | 35.9 | PoNSF | ||
| 60:40 | 15 | A | 98.6 ± 0.6 | 35.9 | PoNSF | ||
| 40:60 | 17.5 | A | 97.8± 0.5 | 35.9 | PoNSF | ||
| 20:80 | 19 | C | - | - | PoNSF | ||
| 10:90 | 20.5 | C | - | - | PoNSF | ||
| 05:95 | 21 | C | - | - | PoNSF | ||
| IND–LYS | Ethanol + water | 95:05 | 1.5 | C | - | - | SF |
| 90:10 | 2.8 | C | - | - | SF | ||
| 80:20 | 7 | A | 100.8 ± 1.2 | 41.8 | SF | ||
| 60:40 | 8.5 | A | 98.9 ± 0.8 | 41.8 | SF | ||
| 40:60 | 10 | A | 102.1 ± 0.7 | 41.8 | SF | ||
| 20:80 | 11.2 | A | D: 39.6 ± 0.4, 97.5 ± 1.2 | - | SF | ||
| 10:90 | 11.8 | C | - | - | SF | ||
| 05:95 | 13 | C | - | - | SF |
| Chemical Substances | Density (g/cm3) | Molar Mass (g/mol) | Water Solubility (mg/mL) | pKa |
|---|---|---|---|---|
| Indomethacin | 1.379 | 357.79 | 0.75 a | 4.5 |
| Arginine | 1.325 | 174.2 | 50 | 12.48 |
| Histidine | 1.412 | 155.15 | 41.6 | 6.04 |
| Lysine | 1.237 | 146.2 | 100 | 10.79 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, J.; Rades, T.; Löbmann, K.; Grohganz, H. Influence of Solvent Composition on the Performance of Spray-Dried Co-Amorphous Formulations. Pharmaceutics 2018, 10, 47. https://doi.org/10.3390/pharmaceutics10020047
Mishra J, Rades T, Löbmann K, Grohganz H. Influence of Solvent Composition on the Performance of Spray-Dried Co-Amorphous Formulations. Pharmaceutics. 2018; 10(2):47. https://doi.org/10.3390/pharmaceutics10020047
Chicago/Turabian StyleMishra, Jaya, Thomas Rades, Korbinian Löbmann, and Holger Grohganz. 2018. "Influence of Solvent Composition on the Performance of Spray-Dried Co-Amorphous Formulations" Pharmaceutics 10, no. 2: 47. https://doi.org/10.3390/pharmaceutics10020047
APA StyleMishra, J., Rades, T., Löbmann, K., & Grohganz, H. (2018). Influence of Solvent Composition on the Performance of Spray-Dried Co-Amorphous Formulations. Pharmaceutics, 10(2), 47. https://doi.org/10.3390/pharmaceutics10020047